5.3b: Low pH Methods
- Page ID
- 48875
5.3b Low pH Methods
The mechanism for low pH treatments is the hydrolysis of hemicellulose. Hydrolysis is a reaction with water, where acid is added to the water to accelerate the reaction time. Several acids can be used, including dilute sulfuric acid (H2SO4), gaseous sulfur dioxide (SO2), hydrochloric acid (HCl), phosphoric acid (H3PO4), and oxalic acid (C2H2O4). Because it is a reaction, the key parameters affecting it include temperature, time, acid concentration, and moisture content of the biomass. The following reactions can take place: hemicellulose can be solubilized, lignin can be separated, acetyl groups are removed, and the surface of the biomass becomes more accessible. As an example (Figure 5.16), the α-1,4 bond is broken by the water and acid to yield two glucose units. An enzyme, amylase, can also promote the reaction. The addition of acid and elevated temperature increases the rate of reaction.
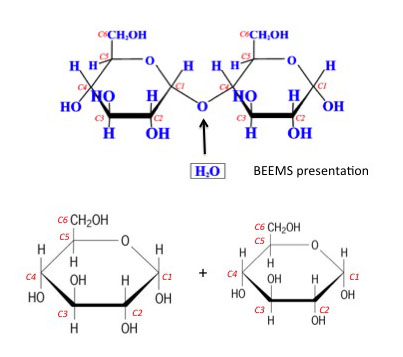
Not only is acid used to facilitate hydrolysis, but acid-catalyzed dehydration of sugars can form furans, which can break down into organic acids such as formic acid and levulinic acid. These compounds can be toxic to the enzymes that are used in sugar fermentation. So after reaction, the residual acid must be neutralized, and inhibitors formed or released during pretreatment must be reduced. Two methods are to use calcium oxide (also known as overliming) or ammonium hydroxide.
Calcium oxide is cheap, forms gypsum during the process, and has a loss of sugar of ~10%, with the necessity of by-product removal and disposal. The reaction is shown below in Reaction 1
Reaction1: CaO + H2SO4→H2O + CaSO4
The advantage of using ammonium hydroxide is that less sugar is lost and less waste is generated, but the cost is higher. The reaction is shown below in Reaction 2.
Reaction 2: 2NH4OH + H2SO4→2H2O + (NH4)2SO4
Figure 5.17a and 5.17b show process diagrams of typical configurations and reaction conditions for sulfuric acid and SO2.
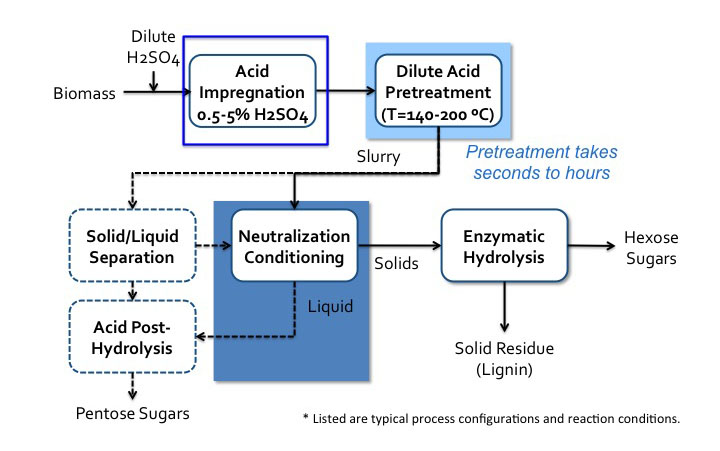
- Click here for a text alternative to figure 5.17a
-
Schematic of sulfuric acid pretreatment process. Biomass is added to dilute sulfuric acid, called acid impregnation (0.5 – 5.0% H2SO4) it then goes to dilute acid pretreatment around (140-200 C). This pretreatment can take seconds to hours. It then becomes a slurry which goes into neutralization conditioning. The solids go to enzymatic hydrolysis where hexose sugars and solid residue (lignin) are separated. The liquids from the neutralization condition go to acid post-hydrolysis. The slurry can also go to solid/liquid separation. The solid products of this separation go to neutralization condition and the liquids go to acid post-hydrolysis. Acid post-hydrolysis yields pentose sugars.
Credit: BEEMS Module B1
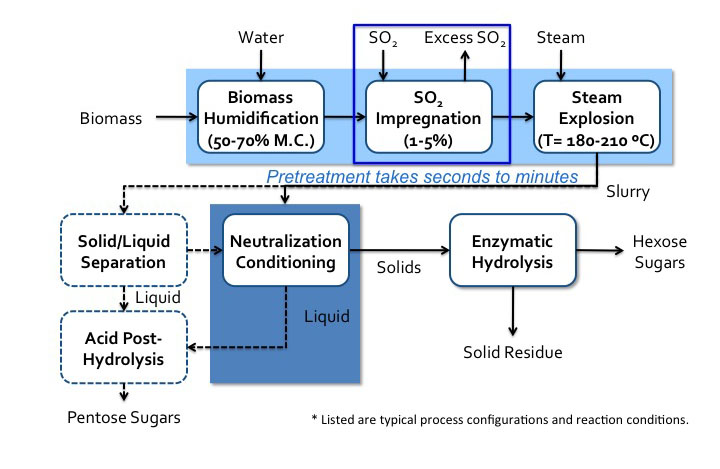
- Click here for a text alternative to figure 5.17b
-
Schematic of sulfur dioxide pretreatment process. Biomass with water undergoes biomass humidification (50%-70% M.C.) and then it undergoes SO2 impregnation (1-5%) and finally a steam explosion (180-210 C). This pretreatment can take seconds to minutes. It then becomes a slurry which goes into neutralization conditioning. The solids go to enzymatic hydrolysis where hexose sugars and solid residue are separated. The liquids from the neutralization conditioning go-to acid post-hydrolysis. The slurry can also go to solid/liquid separation. The solid products of this separation go to neutralization conditioning and the liquids go to acid post-hydrolysis. Acid post-hydrolysis yields pentose sugars.
Credit: BEEMS Module B1


