5.3c: Neutral pH pretreatment
- Page ID
- 48876
5.3c Neutral pH Pretreatment
Pretreatment can also take place in neutral pH water. There are two pathways that can occur. One is when acidic compounds are released from acetylated hemicellulose, mainly acetic acid. This is also called autohydrolysis. Water can also dissociate as the temperature and pressure increases to near the supercritical point (approximately 374°C, 3200 psi), into H+ and OH−, and as this happens, the water behaves like an acid/base system. It is done in water without added chemicals, either in liquid hot water, steam explosion, or water near the supercritical point. The key parameters are time, temperature, and moisture content, and the effects are similar to low pH methods. A schematic for liquid hot water processing is shown in Figure 5.18.
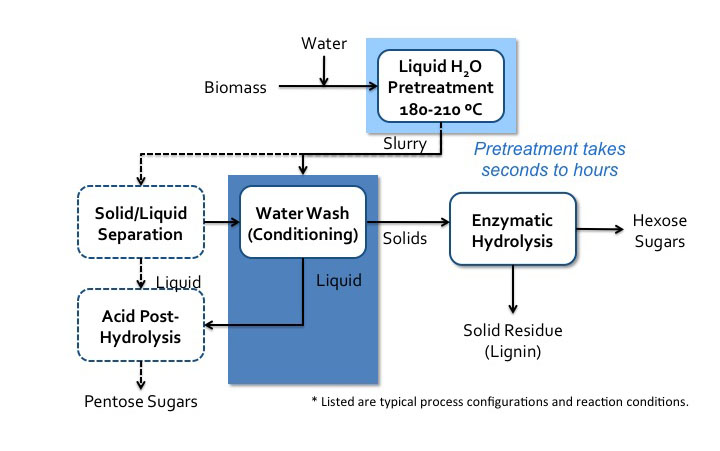
- Click here for a text alternative of Figure 5.18
-
Schematic of liquid hot water prosses flow diagram. Biomass is added to water and sent to liquid H2O pretreatment (180-210 C). Pretreatment can take seconds to hours. It then becomes a slurry which goes into water wash conditioning. The solids go to enzymatic hydrolysis where hexose sugars and solid residue (lignin) is separated. The liquids from the water wash conditioning go to acid post-hydrolysis. The slurry can also go to solid/liquid separation. The solid products of this separation go to water wash conditioning and the liquids go to acid post-hydrolysis. Acid post-hydrolysis yields pentose sugars.
Credit: BEEMS Module B1
One process, developed by Inbicon, is a counter-current multi-stage hot water pretreatment process. There is a pilot-scale unit at Skærbæk, Denmark. It is a three-stage process using hot water (hydrothermal) at 80°C, 160-200 °C, and 190-230°C. After the first stage, liquid composed C5-molasses (sugar) is taken out of the process, which is used for animal feed. After the third stage, the fiber fraction contains cellulose and lignin. Bioethanol and a solid fuel for heat and power are produced when using enzymes, yeast, and fermentation. Figure 5.19 shows the before and after pretreatment of wheat straw (the raw wheat straw and the cellulosic-lignin portion).

Credit: BEEMS Module B1
The next pretreatment processes to discuss are at high pH. The high pH removes the lignin portion of biomass through the breaking of ether linkages (R-O-R’) that hold aromatic phenolic compounds together; ring opening can also take place. It is a depolymerization process. There are several processes and bases used, including: lime, calcium carbonate, potassium hydroxide, sodium hydroxide, and aqueous ammonia. Key parameters include temperature, reaction time, concentration of base, moisture of the feed material, as well as oxidizing agents. The effects include removal of most of the lignin, some removal of hemicellulose, and removal of acetyl links between lignin and hemicellulose.
Lignin is most prominent in grasses and woody biomass. It composes 6-35% of lignocellulosic biomass, depending on the type of grass or wood. Lignin is comprised of crosslinked, branched, monoaromatic units with methoxy and propyl alcohol functional groups. These are shown in Figure 5.20a. Figure 5.20b shows a model of a lignin molecule and how the aromatic monomers are linked together.
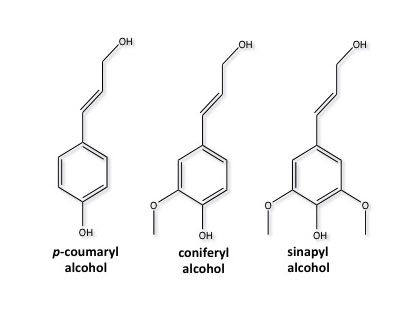
Credit: Bembenic, Meredith, “The chemistry of subcritical water reactions of a hardwood derived lignin and lignin model compounds with nitrogen, hydrogen, carbon monoxide and carbon dioxide,” PhD Thesis, PSU, 2011
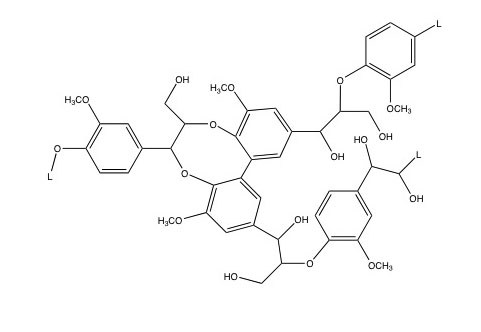
Credit: Bembenic, Meredith, “The chemistry of subcritical water reactions of a hardwood derived lignin and lignin model compounds with nitrogen, hydrogen, carbon monoxide and carbon dioxide,” PhD Thesis, PSU, 2011


