5.3d: High pH (Alkaline)
- Page ID
- 48877
5.3d High pH (Alkaline) Pretreatment
There are two possible outcomes for the chemistry behind the high pH treatment: 1) one is essentially a degradation reaction that liberates lignin fragments and leads to lignin dissolution, and 2) the other is condensation reactions that increase the molecular size of lignin fragments and result in lignin precipitation. As you can see, lignin is a complicated molecule, with a variety of linkages, so reactions are complicated due to lignin complexity. Addition of oxidizing agents greatly improves delignification.
There are multiple processes that have been developed for this type of treatment. Figure 5.21 shows the lime pretreatment process flow diagram. The pretreatment can be done under various conditions, such as oxidative and non-oxidative conditions, short term high temperature (100-200°C, 1-6 h), and long term low temperature (25-65°C, 1-8 weeks). Figure 5.22 shows the soaking in aqueous ammonia (SAA) process flow diagram.
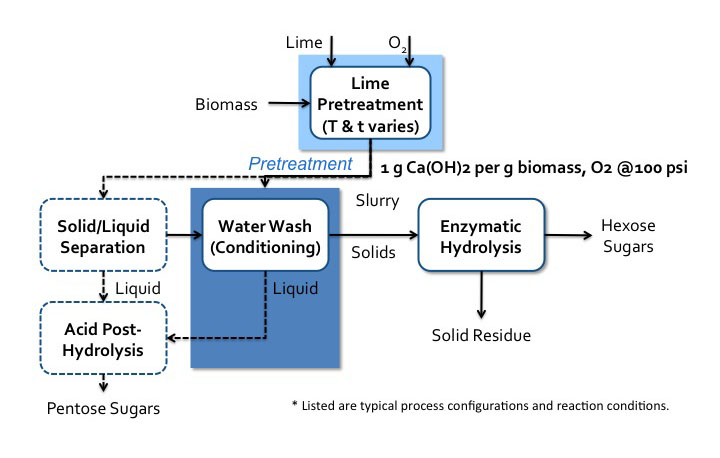
- Click here for a text alternative to Figure 5.21
-
Schematic of the Lime pretreatment process. Biomass enters a lime pretreatment with lime and oxygen gas. The temperatures and time of this process vary. The is about 1g of Ca(OH)2 per 1g of biomass the oxygen gas is at 100psi. After pretreatment, the biomass becomes a slurry that goes into water wash conditioning. The solids go to enzymatic hydrolysis where hexose sugars and solid residue (lignin) is separated. The liquids from the water wash conditioning go-to acid post-hydrolysis. The slurry can also go to solid/liquid separation. The solid products of this separation go to water wash conditioning and the liquids go to acid post-hydrolysis. Acid post-hydrolysis yields pentose sugars.
Credit: BEEMS
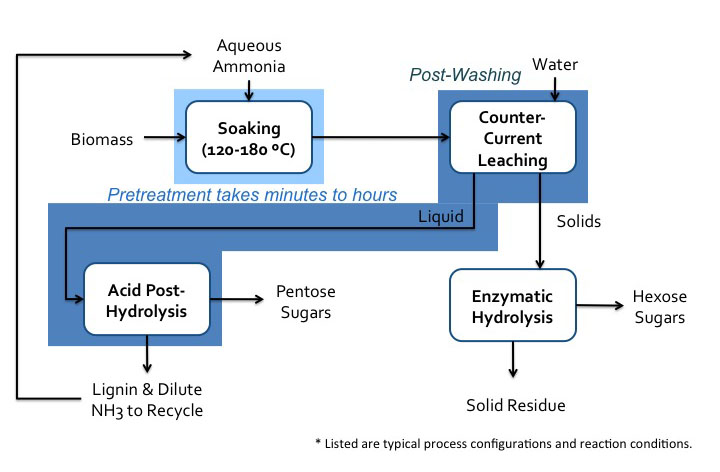
- Click here for a text alternative to Figure 5.22
-
Schematic of soaking in aqueous ammonia (SAA) process. Biomass is soaked in aqueous ammonia at 120-180 C. This pretreatment can take minutes to hours. The biomass then goes to post washing with water and counter-current leaching. The solids go to enzymatic hydrolysis where hexose sugars and the solid residue is separated. The liquids go-to acid post-hydrolysis which removes the pentose sugars and lignin. This also removes the dilute. NH3 which is recycled.
Credit: BEEMS
One of the more developed high pH processes is the ammonia fiber expansion (AFEX) process. Lignocellulosic biomass is soaked in liquid ammonia (causing swelling) followed by the rapid release of pressure (causing expansion). Anhydrous liquid ammonia is used, and key parameters include temperature, residence time, ammonia concentration, and moisture content of the biomass. During this process, there is virtually no compositional change, but lignin is relocated, cellulose is decrystallized, and hemicellulose is depolymerized. This method increases the size and number of micropores in the cell wall to allow for greater accessibility of chemicals for the following stages of processing. A process schematic is shown in Figure 5.23.
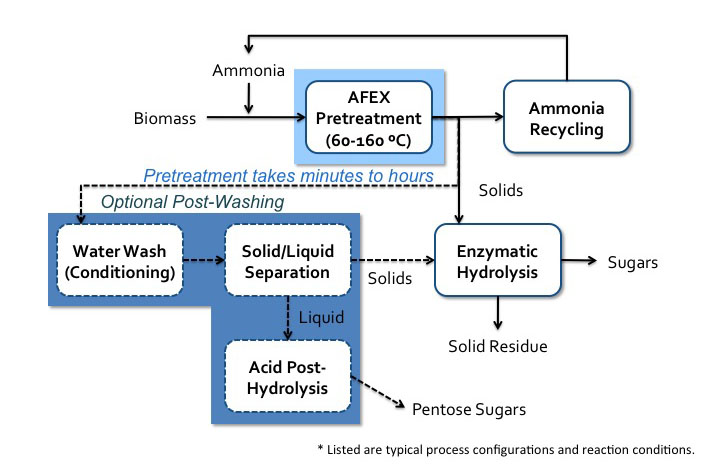
- Click here for a text alternative to Figure 5.23
-
Schematic of AFEX process. Biomass is added with ammonia to AFEX pretreatment (60-160 C). Pretreatment can take minutes to hours. The ammonia is recycled and the solids go to enzymatic hydrolysis where sugars and the solid residue is separated. Before enzymatic hydrolysis, there is an option post-washing step. The solids go into a water wash conditioning and then solid/liquid separation. The solids go into the enzymatic hydrolysis and the liquids go to acid post-hydrolysis which yields pentose sugars.
Credit: BEEMS


