7.3b: How Corn is Processed to Make Ethanol
- Page ID
- 48911
7.3b How Corn is Processed to Make Ethanol
The process of making corn into ethanol is a multistep process. The first step is to milling the corn. It can be done by dry milling or wet milling. Figures 7.10a and 7.10b show the process steps for each wet and dry milling. For wet milling, the corn kernels are broken down into starch, fiber, corn germ, and protein by heating in the sulfurous acid solution for 2 days. The starch is separated and can produce ethanol, corn syrup, or food-grade starch. As is noted in Figure 7.10a, the wet milling process also produces additional products including feed, corn oil, gluten meal, and gluten feed. Dry milling is a simpler process than wet milling, but it also produces fewer products. The main products of dry milling are ethanol, CO2, and dried distiller grain with solubles (DDGS). Let's go through each of the steps in the dry grind process. The five steps are: 1) grinding, 2) cooking and liquefaction, 3) saccharification, 4) fermentation, and 5) distillation.
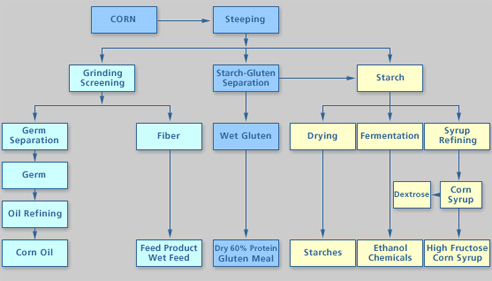
- Click here for a text alternative to figure 7.10a
-
Schematic of The Wet Milling Process
First Corn is steeped. From steeping the corn the products are separated into:Starch/Gluten
The Starch/Gluten goes through a further step of separation and the starch is combined with all the other starch.
The wet gluten is then taken and dried to make dry 60% protein gluten meal
Starch
- The Starch goes through 3 separate processes
- Drying to make starches
- Fermentation to make ethanol chemical
- Syrup refining to make corn syrup, dextrose, and high fructose corn syrup
Corn Germ/Fiber go through grinding screening to yield
- Germ
- goes through oil refining to become corn oil
- Fiber
- Becomes feed product, wet feed
- The Starch goes through 3 separate processes
Credit: Renewable Fuels Association
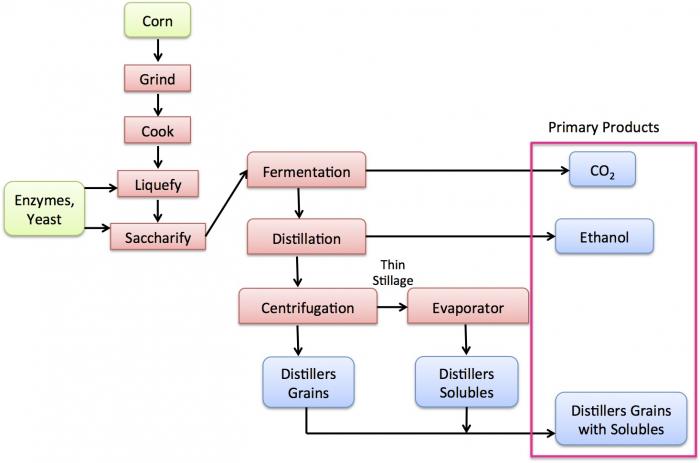
- Click here for a text alternative to figure 7.10b
-
First corn is ground, cooked, liquefied and saccharified. From saccharification, it goes through fermentation which produces CO2. After fermentation, it goes into distillation which produces ethanol. It is then centrifuged and evaporated which yields distillers grains with solubles.
Credit: Caroline Clifford
Grinding
For dry grinding corn, a hammermill or roller mill is used to do the grinding. Figure 7.11 is a schematic of a hammermill with corn being put through it. The hammers are attached to rods that turn on a rotor. As the rotor turns, the feed (corn in this case) is hammered against the wall. A screen at the bottom allows particles that are small enough to leave the unit and keep in the larger particles to continue to be hammered until all the material is in the correct size range. The grinding helps to break the tough outer coatings of the corn kernel, which will increase the surface area of the starch. Once the corn is broken down, it is mixed/slurried with heated water to form a mash or slurry.
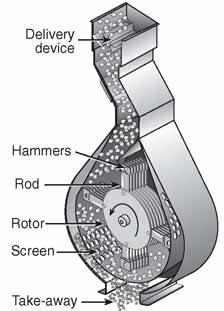
Credit: Feed Machinery
Cooking and Liquefaction
Once the corn slurry (mash) is made, it goes through cooking and liquefaction. The cooking stage is also called gelatinization. Water interacts with the starch granules in the corn when the temperature is >60°C and forms a viscous suspension. Have you ever cooked with cornstarch to make thick gravy? Figure 7.12 shows a picture of starch mixed with water being poured into a heated sauce as it cooks. It will thicken with heat.

Credit: I Want to Cook Blog
The liquefaction step is actually partial hydrolysis that lowers the viscosity. It is essentially breaking up the longer starch chains into smaller chains. One way to measure this is to look at dextrose equivalents (DE), or a measure of the amount of reducing sugars present in a sugar product, relative to glucose, expressed as a percentage on a dry basis. Dextrose is also known as glucose, and dextrose equivalent is the number of bonds cleaved compared to the original number of bonds. The equation is:
\(\text{Equation 1:100} × \dfrac{\text{number of bonds cleaved}}{\text{number of original bonds}}\)
Pure glucose (dextrose): DE = 100
Maltose: DE = 50
Starch: DE = 0
Dextrins: DE = 1 through 13
Dextrins are a group of low molecular weight carbohydrates produced by hydrolysis of starch or glycogen. Dextrins are mixtures of polymers of D-glucose units linked by α (1,4) or α (1,6) glycosidic bonds. Dextrins are used in glues and can be a crispness enhancer for food processing.
Maltodextrin: DE = 3 through 20
Maltodextrin is added to beer.
Recall that starch hydrolysis is where water reacts with the sugar to break the sugar down and form glucose. The water breaks into the H+ and OH- ions to interact with the starch as it breaks down.
In order to accomplish liquefaction, the reaction must take place under certain conditions. The pH of the mash is maintained in the range of 5.9-6.2, and ammonia and sulfuric acid are added to the tank to maintain the pH. About one-third of the required type of enzyme, α-amylase, can be added to the mash before jet cooking (2-7 minutes at 105-120°C) to improve the flowability of the mash. The jet cooking serves as a sterilization step to avoid bacterial contamination during the fermentation step later on. At this stage, shorter dextrins are produced but are not yet glucose.
Three types of processes can be utilized for liquefaction. Figure 7.13 shows the three options. Process 1 is where the α-amylase is added and the material is incubated at 85-95°C. Process 2 has the mash in the jet cooker at 105-120ºC for 2-7 minutes, then flows to a flash tank at 90°C. α-Amylase is added three hours later. The third option, Process 3, adds the α-amylase, the heats in the jet cooker at 150°C, followed by flow to the flash tank at 90°C and adding more α-amylase.
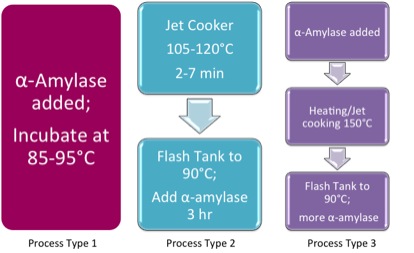
- Click here for a text alternative to figure 7.13
-
Three processes
Process Type 1
α-amylase added; incubated at 85-95ºC
Process Type 2
Jet cooker 105-120ºC for 2-7 minutes
Flash Tank to 90ºC; add α-amylase for 3 hours
Process Type 3
α-amylase added
Heating/Jet cooking @ 150ºC
Flash tank to 90ºC ; add more α-amylase
Credit: BEEMS Module B5
The α-amylase for liquefaction acts on the internal α (1,4) glycosidic bonds to yield dextrins and maltose (glucose dimers). A type of α-amylase exists in the saliva of humans; a different α-amylase is utilized by the pancreas. Figure 7.14a shows one type of α-amylase. The α-amylase works a little faster than the β-amylase, and the β-amylase works on the second α (1,4) glycosidic bond so that maltose is formed (see Figure 7.14b). β-amylase is part of the ripening process of fruit increasing the sweetness of fruit as it ripens.
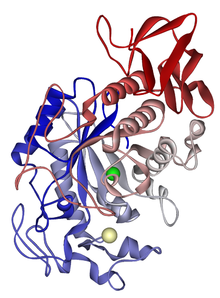
- Click here for a text alternative to figure 7.14a
-
Coming
Credit: Amylase: from Wikipedia.org
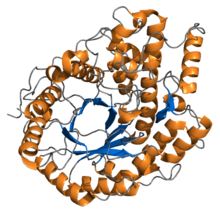
Credit: Amylase: from Wikipedia.org
Saccharification
The next step in the process of making ethanol is saccharification. Saccharification is the process of further hydrolysis to glucose monomers. A different enzyme is used, called a glucoamylase (also known by the longer name amyloglucosidase). It cleaves both the α (1,4) and α (1,6) glycosidic bonds from dextrin ends to form glucose. The optimum conditions are different from the previous step and are at a pH of 4.5 and a temperature of 55-65°C. Figure 7.14c shows a schematic of the glucoamylase, which is also called a ϒ-amylase. There are a wide variety of amylase enzymes available that are derived from bacteria and fungi. Table 7.2 shows different enzymes, their source, and the action of each.
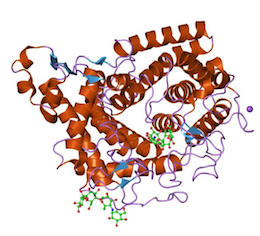
Credit: Global Healing Center
| Enzyme | Source | Action |
|---|---|---|
| α-Amylase | Bacillus amyloliquefaciens | Only α-1,4-oligosaccharide links are cleaved to give a-dextrins and predominantly maltose (G2), G3, G6, and G7 oligosaccharides |
| B. licheniformis | Only α-1,4-oligosaccharide links are cleaved to give a-dextrins and predominantly maltose, G3, G4, and G5 oligosaccharides | |
| Aspergillus oryzae, A. niger | Only α-1,4 oligosaccharide links are cleaved to give a-dextrins and predominantly maltose and G3 oligosaccharides | |
| Saccharifying a-amylase | B. subtilis (amylosacchariticus) | Only α-1,4-oligosaccharide links are cleaved to give a-dextrins with maltose, G3, G4 and up to 50% (w/w) glucose |
| β-Amylase | Malted barley | Only α-1,4-links are cleaved, from non-reducing ends, to give limit dextrins and b-maltose |
| Glucoamylase | A. niger | α-1,4 and α-1,6-links are cleaved, from the nonreducing ends, to give β-glucose |
| Pullulanase | B. acidopullulyticus | Only α-1,6-links are cleaved to give straight-chain maltodextrins |
Some of the newer developed enzymes (granular starch hydrolyzing enzymes – GSHE) allow skipping the liquefaction stage by hydrolyzing starch at low temperatures with cooking. Advantages include: 1) reduced heat/energy, 2) reduced unit operation (reducing capital and operating costs), 3) reduced emissions, and 4) higher DDGS. They work by “coring” into starch granules directly without the water swelling/infusion. Disadvantages include: 1) enzymes cost more and 2) contamination risks.
Fermentation
The final chemical step in producing ethanol from the starch is fermentation. The chemical reaction of fermentation is where 1 mole of glucose yields 2 moles of ethanol and 2 moles of carbon dioxide. The reaction is shown in Equation 2 below:
C6H12O6→2C2H6OH + 2CO2
To cause fermentation to take place, yeast is added. A common yeast to use is saccharomyces cerevisiae, which is a unicellular fungus. The reaction takes place at 30-32°C for 2-3 days in a batch process. Supplemental nitrogen is added as ammonium sulfate ((NH4)2SO4) or urea. A protease can be used to convert proteins to amino acids to add as an additional yeast nutrient. Virginiamycin and penicillin are often used to prevent bacterial contamination. The carbon dioxide produced also lowers pH, which can reduce the contamination risk. Close to 90-95% of the glucose is converted to ethanol.
It is possible to do saccharification and fermentation in one step. It is called Simultaneous Saccharification and Fermentation (SSF), and both glucoamylase and yeast are added together. It is done at a lower temperature than saccharification (32-35°C), which slows the hydrolysis into glucose. As glucose is formed, it is fermented, which reduces enzyme product inhibition. It lowers initial glucose concentrations, lowers contamination risk, lowers energy requirements, and produces higher yields of ethanol. Because SSF is done in one unit, it can improve capital costs and save residence time.
Distillation and Increase of Ethanol Concentration
The last phase of ethanol production is the processing of ethanol to increase the ethanol concentration. Downstream from the fermenters, the ethanol concentration is 12-15% ethanol in water (which means you have 85-88% water in your solution!). Distillation was mentioned in an earlier lesson; crude oil must be distilled into various boiling fractions to separate the oil into useable products. Distillation is a process to separate components using heat and specially designed towers to keep the liquid flowing downward and the vapors being generated to flow upwards. Water boils at 100°C, while ethanol boils at 78°C. However, because water and ethanol evaporate at a lower temperature than their boiling points, and because they both have OH functional groups that are attracted to each other, ethanol and water molecules are strongly bound to each other and form an azeotrope together. That just means that you cannot completely separate ethanol from water – the ethanol fraction will contain about 5% water and 95% ethanol when you get to the end of the distillation process. Figure 7.15 shows a schematic of a distillation unit. You don’t want water in gasoline as you drive, because it prevents efficient combustion. Do you want water in your ethanol if you use it as a fuel?
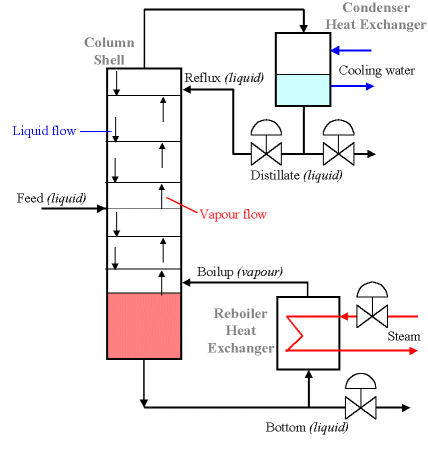
Credit: Newcastle
The answer is no, so you must use an additional method to remove all the water from ethanol. The method is called dehydration. The unit that is used is called a molecular sieve, and the material used in it is called zeolite. Under these conditions, the zeolite absorbs the water into it, but the ethanol will not go into the zeolite. They use what is called a pressure-swing adsorption unit. The unit is designed to run in two modes. At high pressure, the ethanol is dehydrated in Unit 1, and at low pressure, anhydrous ethanol is fed through to remove the water from Unit 2 (Figure 7.16a). When the zeolite sieve has absorbed all the water, Unit 1 is switched to become the low pressure regenerating bed, and Unit 2 becomes the high-pressure unit (Figure 7.16b). The residence time for the process is 3-10 minutes. The zeolite for this process is a highly ordered aluminosilicate with well-defined pore sizes that are formed into beads or included in a membrane. The zeolites attract both water and ethanol, but the pore sizes are too small to allow the ethanol to enter. As noted in Figure 7.17, the pore size of the zeolite membrane is 0.30 nm, while the size of the water molecule is 0.28 nm and the ethanol 0.44 nm. Depending on the type of unit, the membrane or beads can be regenerated using heat and vacuum, or by flowing the pure ethanol through the unit as well as described above.
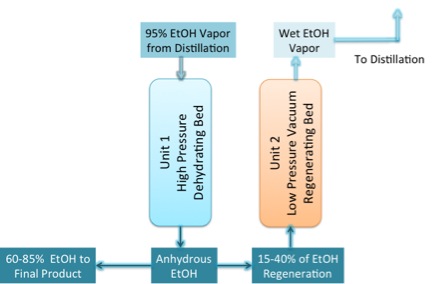
- Click here for a text alternative to figure 7.16a
-
The diagram shows 95% EtOH vapor from distillation going into Unit 1: a high pressure dehydrating bed. Out of that 60-85%, EtOH goes to the final product while 15-40% of the EtOH enters unit 2, a low-pressure vacuum regenerating bed. Out of this, the wet EtOH Vapor goes back to distillation.
Credit: BEEMS Module B5
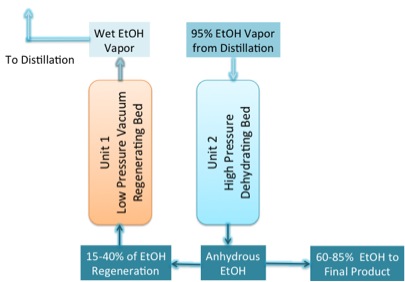
Credit: BEEMS Module B5
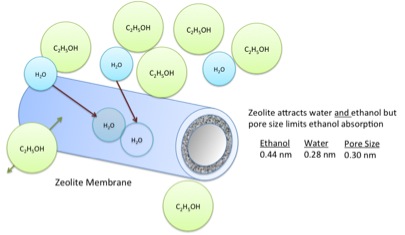
Credit: BEEMS Module B5
So once we have fermented the material to ethanol, it goes through a series of processes to obtain the products in the form that we want them. Figure 7.18a is a schematic of product recovery, and Figure 7.18b shows the definitions of some of the terminology.
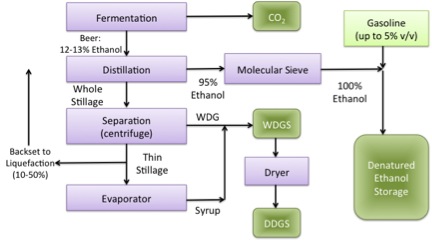
- Click here for a text alternative to figure 7.18a
-
Product recovery diagram of ethanol and other products. From fermentation, CO2 is recovered along with Beer: 12-13% Ethanol. From there distillation occurs. This recovers 95% ethanol which goes through a molecular sieve to become 100% ethanol and goes into denatured ethanol storage with gasoline. From distillation whole stillage is also recovered. This goes into separation/centrifugation and yields thin stillage and WDG. The thin stillage is either recycled or it goes into the evaporator and becomes syrup. The WDG and syrup are combined to become WDGS. The WDGS goes into the dry and becomes DDGS.
Credit: BEEMS Module B5
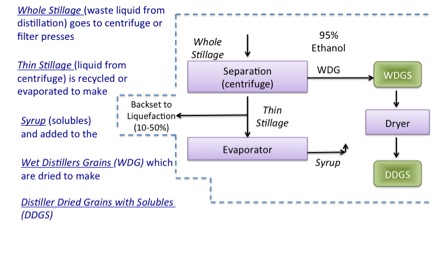
- Click here for a text alternative to figure 7.18b
-
The image defines terminology.
Whole Stillage (waste liquid from distillation) goes to centrifuge or filter presses.
Thin Stillage (liquid from centrifuge) is recycled or evaporated to make
Syrup (solubles) which are added to the
WDG (Wet distillers grains) which are then dried to make
DDGS (Distiller Dried Grains with Solubles)
Credit: BEEMS Module B5
To summarize, corn has 62% starch, 19% protein, 4% oil, and 15% water. If you look at the products on a dry basis (you don’t look at the water like a product), 73% of the corn is starch and 27% is protein, fiber, and oil. For every bushel of corn, realistically you’ll generate 2.8 gallons of ethanol, ~17 lbs of CO2, and ~17 lbs of DDGS. We’ll look at the economics of this process and a couple of other processes in a later lesson.
So, at this point, you can see how to generate ethanol from corn. If you want to generate ethanol from cellulose in plants, you have the information from Lesson 6 to generate glucose from cellulose (it is a more involved process), but once you have glucose, you can use the same end steps in ethanol production from fermentation of glucose. In the next section, we’ll look at the production of another alcohol, butanol.


