Polymer Chemistry: Polymer Crystallinity
- Page ID
- 2921
Most small molecule behavior can be understood in terms of three states: gas, liquid, and solid. Polymers are large molecules with strong intermolecular forces and tangled chains, and do not have a vapor phase - they decompose before the temperature gets high enough to form a vapor. The length of polymer molecules also makes it difficult for the large crystals found in the solid phases of most small molecules to form. Instead solid polymers can be modeled in terms of two phases - crystalline and amorphous. Thus the behavior of polymers can better be understood in term of the three phases: melt, crystalline, and amorphous15.
Crystalline Polymers
Highly crystalline polymers are rigid, high melting, and less affected by solvent penetration. Crystallinity makes a polymers strong, but also lowers their impact resistance. As an example, samples of polyethylene prepared under high pressure (5000 atm) have high crystallinities (95 - 99%), but are extremely brittle21. Small molecules and ions form a three-dimensional lattice with an extended regular structure that makes large crystals possible. A small portion of the NaCl lattice is modeled in the diagram below.

We usually describe such lattices with a unit cell - the smallest repeating unit in the lattice . In the case of NaCl, the unit cell is said to be face-centered cubic.
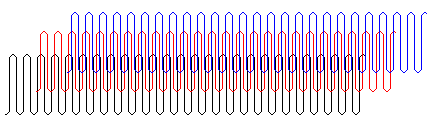
Polymer molecules are very large so it might seem that they could not pack together regularly and form a crystal. It now is known that regular polymers may form lamellar (plate-like) crystals with a thickness of 10 to 20 nm in which the parallel chains (shown in different colors in the simulated structure at the right) are perpendicular to the face of the crystals18.
Amorphous Polymers
Polymer chains with branches or irregular pendant groups cannot pack together regularly enough to form crystals. These polymers are said to be amorphous. A two-dimensional schematic of an amorphous polymer is shown below.
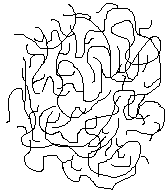
Amorphous regions of a polymer are made up of a randomly coiled and entangled chains. They have been compared to a bucket containing a large number of entangled worms - each one 20-feet long and of 1/4-inch thickness1. The worms are so tangled that an entire worm cannot slide past the others, but small portions of the worms can twist around within the mass. Amorphous polymers are softer, have lower melting points, and are penetrated more by solvents than are their crystalline counterparts.
Glassy and Rubber States
At low temperatures molecular motion in an amorphous region is restricted to molecular vibrations, but the chains cannot rotate or move in space (the worms are frozen and cannot move). This form is the glassy state of the amorphous region. The glassy state can be thought of as being a supercooled liquid where the molecular motions have been frozen in. The glassy state is hard, rigid, and brittle like a crystalline solid, but retains the molecular disorder of a liquid33.
When the material is heated the polymer will reach a temperature at which segments (20 - 50 atoms long) of the entangled chains can move (small portions of the entangled worms can move around so the mass in the bucket writhes). At this temperature, called the glass transition temperature, the amorphous region becomes rubbery. When an amorphous polymer is in its rubbery state it is soft and flexible.
Semi-Crystalline Polymers
Semi-crystalline polymers have both crystalline and amorphous regions. Semi-crystallinity is a desirable property for most plastics because they combine the strength of crystalline polymers with the flexibility of amorphous. Semi-crystalline polymers can be tough with an ability to bend without breaking.
Isolated lamellar single crystals are obtained by crystallization from dilute solution. When crystals are formed from the melt, chain entanglements are extremely important19. In this case the solid is more irregular with polymer chains meandering in and out of ordered crystalline portions. The crystalline portion is in the lamellae; the amorphous portion is outside the lamallae.
Polymers such as this are said to be semi-crystalline. The crystals are small and connected to the amorphous regions by polymer chains so there may be no sharp well-defined boundaries between the two types of regions. For some polymers, such as polyvinyl alcohol, there is a fairly distinct separation between the crystalline and amorphous regions, though. In other cases (e.g., HDPE) the structure basically is crystalline with uniformly-distributed flaws and imperfections20.
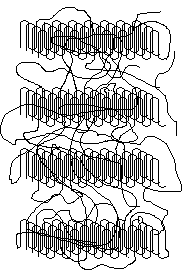
If we model a polymer as having distinct crystalline and amorphous regions then the percentage of the polymer that is crystalline is called the percent crystallinity. The percent crystallinity has an important influence on the properties of the polymer.
Spherulites
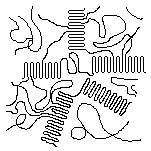
When a molten crystallizable polymer cools, the crystals grow from individual nuclei25 and radiate out like the spokes of a bicycle wheel as is illustrated in the schematic diagram at the right. The crystalline portions actually radiate out in in three dimensions, forming spheres that are called spherulites. In a sample of a crystalline polymer there are billions of spherulites.
Contributors and Attributions
David Whisnant (Wofford College). Partial support for this work was provided by the National Science Foundation's Division of Undergraduate Education through grants DUE #9950809 and DUE #9950296. Additional support was provided by the Camille and Henry Dreyfus Foundation.


