4. Covalent Bonds
- Page ID
- 5935
Solid materials are formed because the bonding between atoms forms a rigid structure. When atoms bond in solids, they form a covalent bond, which means that one or more electrons on a given atom are shared with neighboring atoms.
Here are some points to understand about bonding:
- The electrons on the outermost orbital of an atom are called the valence electrons, but there can only be so many electrons on each orbital; therefore, these valence electrons can either completely fill the “spots” on this orbital, or they can only partially fill the “spots”.
- How many available “spots” or vacancies there are on the valence shell (outermost orbital) determines the ability of a certain atom to bond with others.
Think about it this way: say we have a group of atoms, and they all have the same number of atoms in their valence shells. If they all have their valence shells completely filled (like the noble gases on the Periodic Table), then they are non-reactive and won’t bond covalently. However, if they all have half-filled valence shells, then they will all bond to each other because they will all want to “share” each other’s electrons. By “sharing”, we mean that the atoms will attract the shared electrons equally.
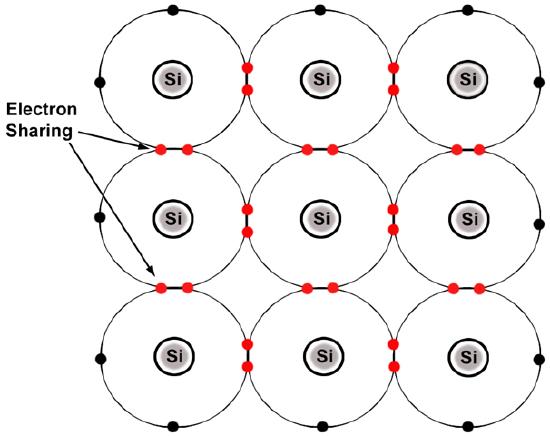
Above we see that silicon (Si) atoms all have half-empty valence shells, so these electrons are actually shared between neighboring silicon atoms.
Source: “Figure 1.4. Silicon atoms form covalent bonds.” Matec.org. 10 March 2012. <matec.org/ps/library3/secure/...1/M092LA1.html>

