9.6: Fuel Cells
- Page ID
- 18996
Components of Fuel Cells and Fuel Cell Systems
A fuel cell is a device which converts chemical energy to electrical energy through the oxidation of a fuel. Like batteries, all fuel cells contain an anode from which electrons and ions flow away, a cathode from which electrons and ions flow towards, and an electrolyte. The electrodes are typically porous which makes it easier for the fuel and oxidizer to get to the reaction site, provides more surface area for the reaction to occur, allows for a higher current through the electrode, and allows for less catalyst to be used [60, ch. 5]. The electrolyte may be a liquid or a solid. Examples of liquid electrolytes include potassium hydroxide solution and phosphoric acid solution [128]. Examples of solid electrolytes include (ZrO\(_2\))\(_{0.85}\)(CaO)\(_{0.15}\) and (ZrO\(_2\))\(_{0.9}\)(Y\(_2\)O\(_3\))\(_{0.1}\) [60]. Also like a battery, individual cells may be stacked together in a package. A single fuel cell may have a cell voltage of a few volts, but multiple cells may be packaged together in series to produce tens or hundreds of volts from the unit.
| Fuel cell components |
|---|
| Anode |
| Cathode |
| Electrolyte |
| Membrane |
| Catalyst |
| Fuel |
| Oxidizer |
| Fuel cell system components |
|---|
| Fuel processor |
| Flow plates |
| Heat recovery system |
| Inverter |
| Other electronics |
In addition to these components, fuel cells often contain a thin polymer membrane, and fuel cell electrodes are often coated with a catalyst which speeds up the chemical reaction. An example material used to make the membrane is a 0.076 cm layer of polystyrene [60, ch. 10]. Another example membrane is polybenzimidazole containing phosphoric acid [128, ch. 37]. Membranes allow ions, but not the fuel and oxidizer to pass through [60, ch. 10]. In addition to selectively allowing ions to pass through, membranes should be chemically stable to not break down in the presence of the often acidic or alkaline electrolyte, should be electrical insulators, and should be mechanically stable [60, ch. 10]. A useful catalyst speeds up the reaction at the electrodes. In addition, a good catalyst must not dissolve or oxidize in the presence of the electrolyte, fuel, and oxidizer [60, ch. 8]. Additionally, it should only catalyze the desired reaction, not other reactions [60, ch. 8]. Examples of catalysts used include platinum, nickel, acetylacetone, and sodium tungsten bronze Na\(_x\)WO\(_3\) with \(0.2 < x < 0.93\), [60, ch. 6].
During operation, the fuel and oxidizer are continuously supplied to the device. Fuel may be in the form of a gas such as hydrogen or carbon monoxide gas, it may be in the form of a liquid such as methanol or ammonia, or it may be in the form of a solid such as coal [60, ch. 10]. Oxygen gas or air which contains oxygen is typically used as the oxidizer [60, ch. 10].
Additional chemical, mechanical, thermal, and electrical components are often included in an entire fuel cell system. Some fuel cell systems include a fuel processor which breaks down the fuel to convert it to a usable form and which filters out impurities [141]. For example, a fuel processor may take in coal and produce smaller hydrocarbons which are used as fuel. Also, fuel cells system may contain flow plates which channel the fuel and oxidizer to the electrodes and channel away the waste products and heat [141]. Some fuel cells include heat recovery systems, built in thermoelectric devices which convert some of the heat generated back to electricity. For systems intended to be connected to the electrical grid, inverters which convert the DC power from the fuel cell to AC are included. A fuel cell system also typically include a control system that regulates the flow of the fuel and oxidizer, monitors the temperature of the device, and manages its overall operation [128, ch. 37].
Types and Examples
Fuel cells may be classified in different ways. One way is by operating temperature: low \(25-100 ^{\circ}\)C, medium \(100-500 ^{\circ}\)C, high \(500-1000 ^{\circ}\)C, and very high over \(1000 ^{\circ}\)C [60, ch. 1]. Chemical reactions typically occur more quickly at higher temperatures. However, one challenge of designing high temperature fuel cells is that materials must be selected that can withstand the high temperatures without melting or corroding [60, ch. 2].
As with batteries, another way to classify fuel cells is as primary or secondary [60, ch. 1]. In a primary fuel cell, also called nonregenerative, the reactants are used once then discarded. In secondary fuel cells, also called regenerative, the reactants are used repeatedly. An external source of energy is needed to refresh the fuel for reuse, and this source may supply energy electrically, thermally, photochemically, or radiochemically [60, p. 515]. Both primary and secondary fuel cells have been made with a variety of organic and inorganic fuels [60, p. 515].
Another way to classify fuel cells is as direct or indirect [60, ch. 1,7] [128, ch. 37]. In a direct fuel cell, the fuel is used as is. In an indirect fuel cell, the fuel is processed first inside the system. For example, an indirect fuel cell may take in coal and use an enzyme to break it down into smaller hydrocarbons before the reaction of the cell [60, ch. 7].
Families of fuel cells are often distinguished by the type of electrolyte used. Examples include alkaline which use a potassium hydroxide solution as the electrolyte, phosphoric acid, molten carbonate, and solid oxide which use solid ceramic electrolytes. Other times, fuel cells are categorized by the type of membrane or the type of fuel used. Two of the most common types of fuel cells are proton exchange membrane fuel cells and direct methanol fuel cells [128, ch. 37]. Proton exchange membrane fuel cells use hydrogen gas as the fuel, oxygen from air as the oxidizer, a solid electrolyte, and a platinum catalyst [128] [141]. They operate at low temperature and are used in buses, aerospace applications, and for backup power. Direct methanol fuel cells use methanol as a fuel. They also often operate at low or medium temperatures [128] and are used for similar applications.
Practical Considerations of Fuel Cells
The history of fuel cells goes back almost as long as the history of batteries. The concept of the fuel cell dates to around 1802 [3, p. 2,222] [60, p. v]. Working fuel cells were demonstrated in the 1830s [3, p. 222] [60, p. v], and the first practical device was built in 1959 as pure materials became commercially available [5, p. 46] [60, p. v, 26]. While both batteries and fuel cells are commercially available, batteries have found a home inside almost every every car, computer, and electronic devices while fuel cells are more specialized products. There are a number of limitations of fuel cell technology that have prevented more widespread use. One limitation is their cost. Some fuel cells use platinum as the catalyst, and platinum is not cheap. Some cells that do not use platinum catalysts have the problem that their efficiency is reduced in the presence of carbon monoxide or carbon dioxide, which are commonly found in air. Hydrogen gas or methane are used as the fuel in some cells, and the delivery and storage of these fuels pose challenges. Additionally, some of the more efficient systems are large and require fixed space, air or water cooling, and additional infrastructure, so these devices do not lend themselves to portable applications.
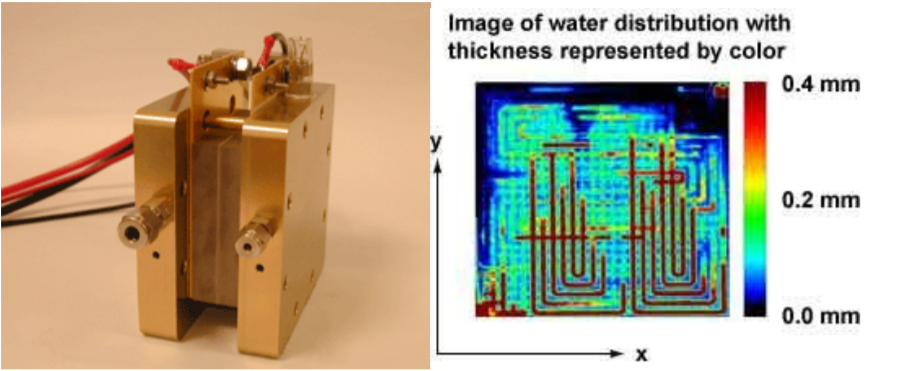
Fuel cells have advantages which lead to useful applications. Many fuel cells produce no harmful outputs. If hydrogen gas is used as the fuel and oxygen from the air is used as the oxidizer, the only byproduct is pure water. It is hard to find an energy conversion device which generates electricity and is easier on the environment than this type of fuel cell. The left part of Fig. \(\PageIndex{1}\) shows a photograph of a proton exchange membrane fuel cell. The right part of Fig. \(\PageIndex{1}\) shows an image of the water formed during its operation. The image was obtained by the neutron radiography method, and it was taken at the National Institute of Standards and Technology Center for Neutron Research in Gaithersburg, Maryland. These figures are used with permission from [150]. In some applications, the water production is a main advantage. NASA space vehicles have used fuel cells to produce both electricity and pure water since the Gemini and Apollo projects dating to the 1960s [3, p. 250]. They have been used to produce both electricity and water on military submarines since the 1960s too [3, p. 250]. Another advantage of fuel cells is that they can be more efficient than other devices which generate electricity. High temperature and higher power units can have efficiencies up to 65% [128]. Since some of the highest efficiencies are achieved in higher temperature and higher power devices, fuel cells have found a niche in large and stationary applications generating kilowatts or megawatts of electricity.