7.2: Devices Involving Spontaneous Emission
- Page ID
- 18980
Spontaneous emission occurs in many commercially available consumer products. This section discusses three categories of devices that convert electricity to light by spontaneous emission: incandescent lamps, gas discharge lamps, and LEDs.
Incandescent Lamps
An incandescent lamp is a device that converts electricity to light by blackbody radiation. These devices are typically constructed from a solid metal filament inside a glass walled vacuum tube. A current passes through the filament which heats it to a temperature of thousands of degrees. High temperatures are used because the visible spectral response of daylight is close to the visible spectral response of a blackbody radiator at a temperature of 6500 K [87]. The main limitation of incandescent lamps is their efficiency. Much of the electromagnetic radiation emitted by a blackbody radiator falls outside the visible range.
The main advantage of incandescent lamps over other technologies is their simplicity. For this reason, incandescent lamps were some of the earliest lamps developed. Humphry Davy demonstrated that blackbody radiation could be used to produce visible light in 1802, and practical incandescent lamps date to the 1850s [88]. In order to develop these practical incandescent lamps, vacuum pumping technology had to be developed, and technology to purify the metal used to make lamp filaments was required [88].
In some ways, an incandescent lamp is similar to an antenna. In both cases, the input takes the form of electricity, and this electrical energy is converted to electromagnetic energy by passing through a conducting wire. In an antenna, the input is time varying to encode information, and the output is at radio or microwave frequencies. However, in an incandescent lamp, the input is typically AC and does not contain information. The desired output of an incandescent lamp is visible light, but it also produces heat and electromagnetic radiation at infrared frequencies and at other non-visible frequencies. Additionally, antennas are typically designed to operate at a wavelength close to the length of the antenna, and such antennas can produce waves with specific electromagnetic polarization and radiation patterns. Spontaneous emission in incandescent lamps, however, is necessarily unpolarized and incoherent.
Gas Discharge Lamps
A gas discharge occurs when a conducting path forms through a plasma, an ionized gas [89]. Gas discharge devices convert electricity to light by spontaneous emission when this type of conducting path forms. In 1802 in addition to demonstrating blackbody radiation and proposing the idea of a fuel cell, Humphry Davy demonstrated a gas discharge device [3, p. 222] [88]. W. Petrov demonstrated a gas discharge around the same time [88]. One of the first practical gas discharge lamps, a carbon arc lamp, was built by Leon Foucoult in 1850, and it was used for theater lighting [88]. Development of gas discharge lamps required the ability to purify gases in addition to the development of vacuum pumping technology [88]. Examples of gas discharge devices in use today include include sodium vapor lamps, mercury arc lamps, fluorescent lamps, and neon advertising signs [89].
A gas discharge lamp is made from a sealed tube containing two electrodes and filled by a gas. The glass tube contains the gas, maintains the gas pressure, and keeps away impurities. The pressure of the gas inside the tube can range from \(10^{-4} Pa\) to \(10^5 Pa\) for different lamps [87, p. 206]. Typical electrode spacing is on the order of centimeters [87]. Some neon bulbs have an electrode spacing of 1 mm while many fluorescent tubes have an electrode spacing over 1 m. Hundreds to millions of volts are applied across the electrodes [89]. Transformers are used to achieve these high voltage levels. The voltage between the electrodes ionizes the gas inside the tube and provides a supply of free electrons which travel along the conducting path between the electrodes [89]. The gas may be ionized, and electrons supplied, by other methods such as chemical reactions, a static electric field, or an optical field instead [87, Ch. 5]. Electrons may also be supplied to the gas by thermionic emission, boiling electrons off the cathode.
The optical properties of the lamp are determined by the gas inside the tube. Energy supplied by the electric field across the electrodes, or other means, excites electrons of the gas atoms to higher energy levels. Spontaneous emission occurs between distinct allowed energy levels only, so the emission occurs over relatively narrow wavelength ranges. Gases are chosen to have allowed energy level transitions in the desired wavelength range. Typical gases used include helium, neon, sodium, and mercury [87, p. 514].
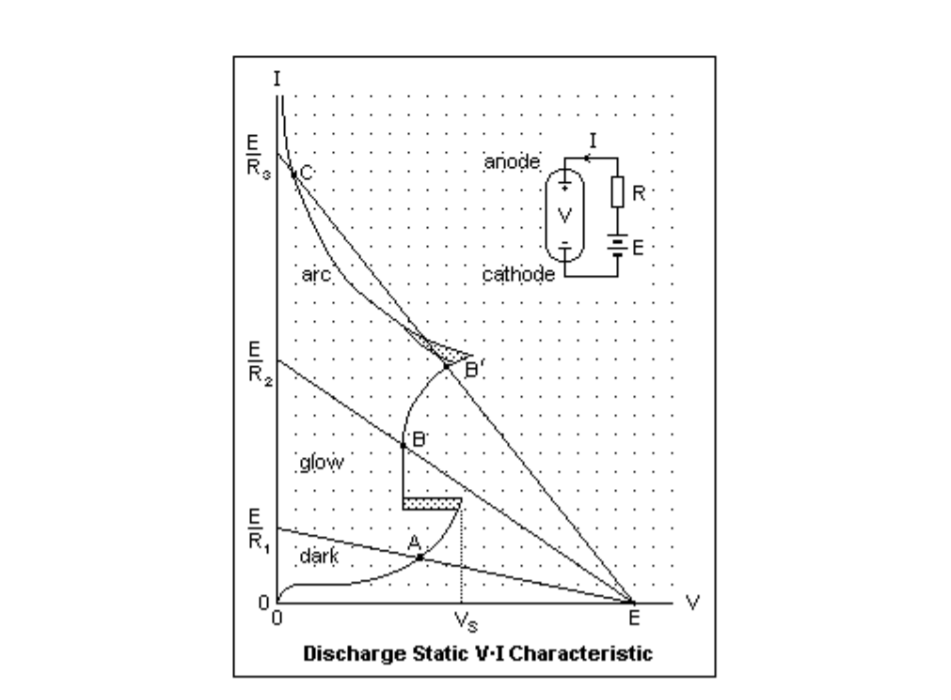
Gas discharge lamps are classified as either glow discharge devices or arc discharge devices. Figure \(\PageIndex{1}\) shows an example plot of the current between electrodes as a function of voltage. As shown in the figure, the currentvoltage characteristic of a gas discharge tube is quite nonlinear. However, it can be broken up into three general regions, denoted the dark region, the glow region, and the arc region. The regions are distinguished by a change in slope of the current-voltage plot. This figure is used with permission from [89] which provides more details on the physics of gas discharges.
The dark region of operation corresponds to low currents and voltages, and devices operating in this region are said to have a dark or Townsend discharge. Optical emission from devices operating in this region are not self sustaining. While atoms of the gas may ionize and collide with other atoms, no chain reaction of ionization occurs. The transition between the dark and glow discharges is called the spark [87, p. 160]. In Fig. \(\PageIndex{1}\), VS is the sparking voltage. The second region, corresponding to higher currents, is called the glow region, and this region is called self sustaining because ions collide and ionize additional gas atoms producing more free electrons in an avalanche process. Significant spontaneous emission occurs in the glow discharge region [87] [89]. The third region, corresponding to even higher current, is called the arc region. Arc discharges are also self sustaining [87, p. 290], and spontaneous emission is produced. Once the arc discharge is established, relatively low voltages are required to maintain it compared to the voltages needed to maintain the glow discharge.
Fluorescent lamps are a type of gas discharge device that involves the use of chemicals with desired optical properties, called phosphors [87, p. 542]. The gas and electrode voltage used in fluorescent lamps is chosen to so that the spontaneous emission produced is at ultraviolet frequencies. These UV photons may be produced by either an arc or glow discharge. The UV photons produced are absorbed by the phosphor molecules, and the phosphor molecules emit light at lower frequencies. Examples of phosphors used include zinc silicate, calcium tungstate, and zinc sulfide [87, p. 542].
LEDs
LEDs are devices that convert electricity to light by spontaneous emission. They are made from pn junctions in semiconductors. Pn junctions were discussed in Section 6.5. When a forward bias is applied across a pn junction, electrons and holes are injected into the junction. The energy from the power supply excites electrons from the valence to the conduction bands. These excited electrons can flow through the material much more easily than unexcited electrons. Some of the electrons and holes near the junction combine and spontaneously emit photons in the process. Some LEDs have a thin intrinsic, undoped, layer between the p-type and n-type layers at the junction to improve efficiency.
LEDs emit light over a relatively narrow range of frequencies. The frequency of light emitted is determined by the energy gap of the semiconductor. Semiconductors are used because the energy gap of semiconductors corresponds to the energy of near ultraviolet, visible, or infrared photons. While light emitted by an LED has a narrow range of frequencies, lasers emit light with a much narrower range of frequencies. LEDs emit light within a narrow frequency range, but applications, such as residential lighting, require white light with a broader bandwidth. One strategy used to produce white light from an LED is to use phosphors. In such a device, an LED converts electricity to near UV or blue light. The phosphors absorb the blue light and emit light at lower energies, at wavelengths in the visible range. For this reason, blue LEDs were particularly important for generating white light. It took decades from the invention of red LEDs in the 1960s until reliable blue LEDs were developed in the 1980s and 1990s. In 2014, Isamu Akasaki, Hiroshi Amano and Shuji Nakamura were awarded the Nobel Prize in physics for their work developing blue LEDs. This effort required the development of deposition technology for new materials like gallium nitride, and it required being able to deposit these materials in very pure layers without mechanical strain tearing the materials apart [90].
A related device which emits light by spontaneous emission is an organic light emitting diode, OLED. In an OLED, a voltage excites electrons in a thin layer, 100-200 nm, of an organic material, and the type of organic material used determines the wavelength of light emitted [91]. Some flat panel displays are made from arrays of OLEDs. White light in these displays is achieved from a combination of red, green, and blue OLEDs near to each other [91].
LEDs are small devices that can often fit into a cubic millimeter. For this reason, they can be integrated into electronics more easily than devices like incandescent lamps and gas discharge lamps which require vacuum tubes. LEDs require low voltages electricity to operate. Since they require a small amount of input electrical power, they produce a small amount of output optical power. Incandescent lamps and gas discharge lamps have advantages in high power applications, but arrays of LEDs can also be used in these applications. Another advantage of LEDs is that they have a longer useful lifetime. In gas discharge lamps, the electrodes sputter, depositing material onto the surface of the tube, limiting the lifetime of the device.


