6.12: Graphene
- Page ID
- 52345
Polyacetylene is a 1d chain of carbon atoms, each contributing one electron in a frontier atomic orbital. It is also possible to form 2d sheets of carbon atoms with a single electron in their frontier atomic orbitals. See for example graphene in Figure 6.13.1.\(^{†}\)
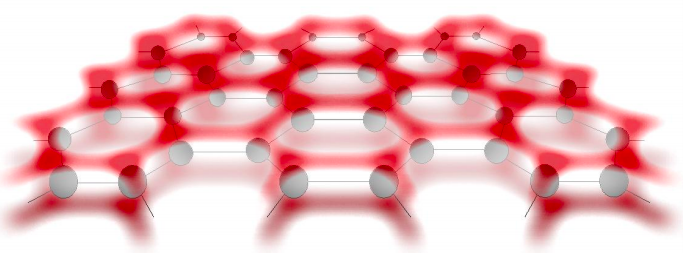
Graphene may also be rolled up into cylinders to form carbon nanotubes – unique structures that we will consider in detail later on in the class.
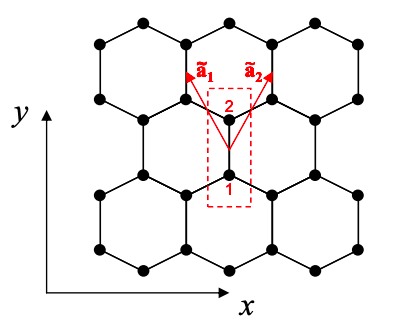
The unit cell of graphene contains two carbon atoms labeled 1 and 2 in Figure 6.13.2. The lattice is generated by shifting the unit cell with the primitive lattice vectors \({\bf{\tilde{a_{1}}}} = a_{0}\left( -\sqrt{3}/2,3/2 \right)\) and \({\bf{\tilde{a_{2}}}} = a_{0}\left(\sqrt{3}/2,3/2 \right)\), where \(a_{0}\) is the carbon-carbon bond length.
\(^{†}\)In graphene extended \(\pi\) orbitals are formed above and below the plane of a sheet of hexagonal carbon atoms, increasing the rigidity of the structure and enhancing charge transport.

