6.16: 2-d and 3-d periodic materials
- Page ID
- 52349
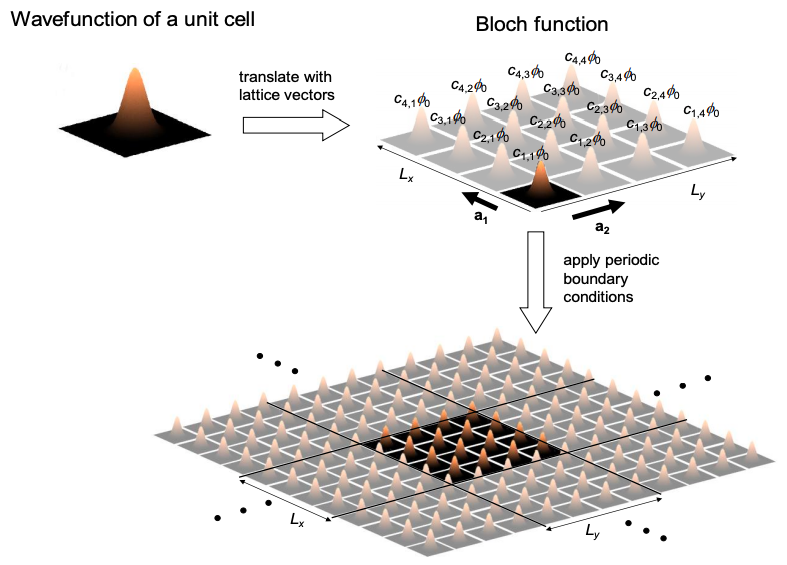
Applying Bloch functions to periodic 2d and 3d molecules follows the same principles as in 1d; see Figure 6.17.1.
The molecular orbitals in 2-d and 3-d periodic materials are still composed of linear combinations of the wavefunction of the unit cell, \(\phi_{0}\), i.e.
\[ \Psi = \sum_{r}c_{r}\phi_{0} \nonumber \]
Once again, when we apply periodic boundary conditions the area occupied in k-space per k-state is: (for 2-d and 3-d, respectively)
\[ \Delta k^{2} = \Delta k_{x}\Delta k_{y} = \frac{2\pi}{L_{x}} \frac{2\pi}{L_{y}} = \frac{4\pi^{2}}{A} , \ \ \ \Delta k^{3} = \Delta k_{x}\Delta k_{y}\Delta k_{z} = \frac{2\pi}{L_{x}} \frac{2\pi}{L_{y}}\frac{2\pi}{L_{z}} = \frac{8\pi^{3}}{V} \nonumber \]
where A is the area of the molecule, and V is its volume.
3-d periodic materials are usually known as crystals. Si and the rest of the common semiconductor materials fall into the category of 3-d periodic materials.