6.1: Composition and Structure of the Atmosphere
- Page ID
- 12258
By the end of this chapter, students will be able to
- Describe the composition and structure of the atmosphere.
- Explain the importance of the ozone layer, its depletion, and specific steps taken to address it.
- Distinguish between indoor and outdoor air pollution and how these compare among industrialized and less industrialized countries
- Identify natural and anthropogenic sources of air pollution
- Explain the effects of air pollution on human and ecosystem health
- Explain how the Clean Air Act legislation works and describe its outcomes
Atmosphere refers to the layer of gases that surrounds Earth and is held in place by Earth’s gravitational attraction (gravity). The mix of gases in the atmosphere forms a complex system organized into layers that together support life on Earth. Although there are numerous gases, as shown in Table \(\PageIndex{1}\), the top four gases make up 99.998 % of the volume of clean dry air (unpolluted air that does not contain water vapor). Of this dry composition of the atmosphere nitrogen, by far, is the most common (78%).
.png?revision=1&size=bestfit&width=549&height=349)
Nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the Earth's surface, as oxygen gas is a necessary reactant of the combustion process. Nitrogen is also needed and used by living things to make proteins, though as nitrogen gas, N2, it is unavailable to most living things. Oxygen is used by all living things to make molecules that are essential for life. It is also essential for aerobic respiration as well as combustion or burning. Argon is a non-reactive gas and we use it in light bulbs, in double-pane windows, and to preserve priceless documents such as the original Declaration of Independence and the Constitution. Carbon dioxide is an essential gas used by plants and other organisms to make sugar (food) through photosynthesis. This process is essential for other life as well because during photosynthesis, water molecules are split apart and their oxygen is released back to the atmosphere. Carbon dioxide also acts as a blanket that prevents the escape of heat into outer space (see more on this in Chapter 7). The atmosphere is rarely, if ever, completely dry. Water vapor (water in a 'gas' state) is usually present up to about 4% of the total volume depending on location. In the Earth's desert regions (30° N/S) when dry winds are blowing, the water vapor contribution to the composition of the atmosphere will be near zero.
| Gas | Symbol | Content |
|---|---|---|
| Nitrogen | N2 | 78.08% |
| Oxygen | O2 | 20.95% |
| Argon | Ar | 0.93% |
| Carbon | CO2 | 0.03% (this and the above three add to 99.998%) |
| Neon | Ne | 18.20 parts per million |
| Helium | He | 5.20 ppm |
| Krypton | Kr | 1.10 ppm |
| Sulfur Dioxide | SO2 | 1.00 ppm |
| Methane | CH4 | 2.00 ppm |
| Hydrogen | H2 | 0.50 ppm |
| Nitrous Oxide | N2O | 0.50 ppm |
| Xenon | Xe | 0.09 ppm |
| Ozone | O3 | 0.07 ppm |
| Nitrogen Dioxide | NO2 | 0.02 ppm |
| Iodine | I2 | 0.01 ppm |
| Carbon Monoxide | CO | Trace |
| Ammonia | NH3 | Trace |
Source: National Weather Service www.srh.noaa.gov/jetstream/at...tmos_intro.htm
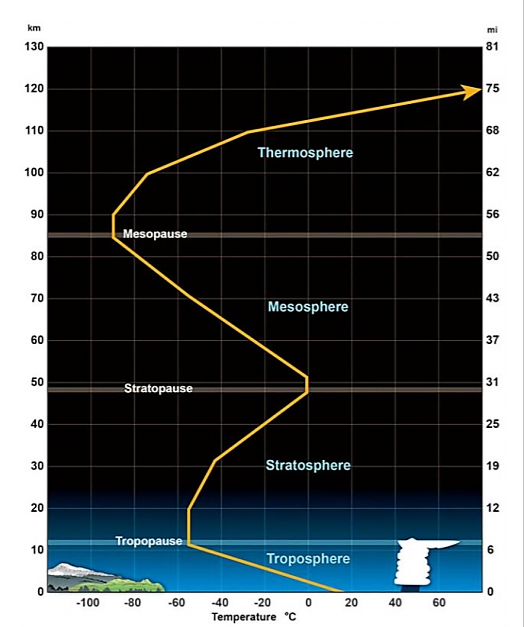
Earth’s atmosphere is divided into four distinct layers based on thermal characteristics (temperature changes), chemical composition, movement, and density (Figure \(\PageIndex{2}\)). The troposphere is the lowest layer extending from the surface up to roughly 18 km above the surface depending on location (varies from as low as 6 km to as high as 20 km). There is continuous flow and swirling of air constantly through convection currents redistributing heat and moisture around the globe. This results in the short-lived and local patterns of temperature and moisture that we call weather. Because gravity holds most air molecules close to the Earth’s surface, the troposphere is the densest of all layers, containing about 75% of the total mass of the atmosphere. The density of the gases in this layer decrease with height so the air becomes thinner. In response, the temperature in the troposphere also decreases with height. As one climbs higher, the temperature drops from an average around 17°C (62°F) at sea level to about -51°C (-60°F) at the tropopause, a sharp boundary at the top of the troposphere that limits mixing between the troposphere and the upper layers.
.png?revision=1&size=bestfit&width=579&height=693)
The stratosphere is the layer that extends from the tropopause up to about 50 km to 53 km above the Earth’s surface depending on location. The proportions of most gases in this layer is similar to that of the troposphere with two main exceptions: 1) there is almost no water vapor in the stratosphere and 2) the stratosphere has nearly 1,000 times more ozone (O3) than the troposphere. With only about 19% of the total mass of the atmosphere, the density of the stratosphere is significantly lower than the troposphere. However, temperature in this region increases with height as a result of heat that is produced during the formation of ozone (more on ozone in section 6.2). This heat is responsible for temperature increases from an average -51 °C (-60°F) at tropopause to a maximum of about -15°C (5°F) at the top of the stratosphere. This increase in temperature with height means warmer air is located above cooler air. This prevents "convection" as there is no upward vertical movement of the gases. The consequence of this little to no mixing of gases in the stratosphere makes it relatively calm but also means that once substances such as pollutants enter this zone, they can remain suspended for many years. The top of the stratosphere is bound by a boundary known as the stratopause.
Above the stratosphere is the mesosphere which extends to about 85 km above the Earth's surface. The mesosphere has no ozone molecules and the other gases such as oxygen and nitrogen continue to become less dense with height. As a result, not much ultraviolet and x-ray radiation from the sun is absorbed by molecules in this layer so temperature decreases with altitude. Both the stratosphere and the mesosphere are considered the middle atmosphere.
Between about 85 km and 600 km lies the thermosphere. This layer is known as the upper atmosphere. Unlike the mesosphere, the gases in this layer readily absorb incoming high energy ultraviolet and x-ray radiation from the sun. Because of this absorption, the temperature in the thermosphere increases with height and can reach as high as 2,000°C (3,600°F) near the top depending on solar activity. However, despite the high temperature, this layer of the atmosphere would still feel very cold to our skin due to the very thin atmosphere. The high temperature indicates the amount of energy absorbed by molecules but with so few in this layer, the total number of molecules is not enough to heat our skin. There’s no sharp boundary that marks the end of the atmosphere. Pressure and density simply continue to decrease with distance until they become indistinguishable from the near-vacuum of outer space.

