1.1: The Chemical Foundations of Life
- Page ID
- 12218
At the end of this section, students will be able to:
- Describe, at an introductory level, the basic chemical and biological foundations of life on Earth
- Define environment, ecosystems, and environmental sciences
- Give examples of the interdisciplinary nature of environmental science
- Define sustainability and sustainable development
- Explain the complex relationship between natural and human systems, pertaining to environmental impact, the precautionary principle, and environmental justifications
- Understand scientific approach and begin to apply the scientific method
The Chemical and Biological Foundations of Life
Elements in various combinations comprise all matter on Earth, including living things. Some of the most abundant elements in living organisms include carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus. These form the nucleic acids, proteins, carbohydrates, and lipids that are the fundamental components of living matter. Biologists must understand these important building blocks and the unique structures of the atoms that make up molecules, allowing for the formation of cells, tissues, organ systems, and entire organisms.
At its most fundamental level, life is made up of matter. Matter is any substance that occupies space and has mass. Elements are unique forms of matter with specific chemical and physical properties that cannot be broken down into smaller substances by ordinary chemical reactions. There are 118 elements, but only 92 occur naturally. The remaining elements are synthesized in laboratories and are unstable. The five elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N) and phosphorous (P). In the non-living world, elements are found in different proportions, and some elements common to living organisms are relatively rare on the earth as a whole (Table \(\PageIndex{1}\)). For example, the atmosphere is rich in nitrogen and oxygen but contains little carbon and hydrogen, while the earth’s crust, although it contains oxygen and a small amount of hydrogen, has little nitrogen and carbon. In spite of their differences in abundance, all elements and the chemical reactions between them obey the same chemical and physical laws regardless of whether they are a part of the living or non-living world.
| Biosphere | Atmosphere | Lithosphere | |
|---|---|---|---|
| Oxygen (O) | 65% | 21% | 46% |
| Carbon (C) | 18% | trace | trace |
| Hydrogen (H) | 10% | trace | trace |
| Nitrogen (N) | 3% | 78% | trace |
| Phosphorus (P) | trace | trace | >30% |
The Structure of the Atom
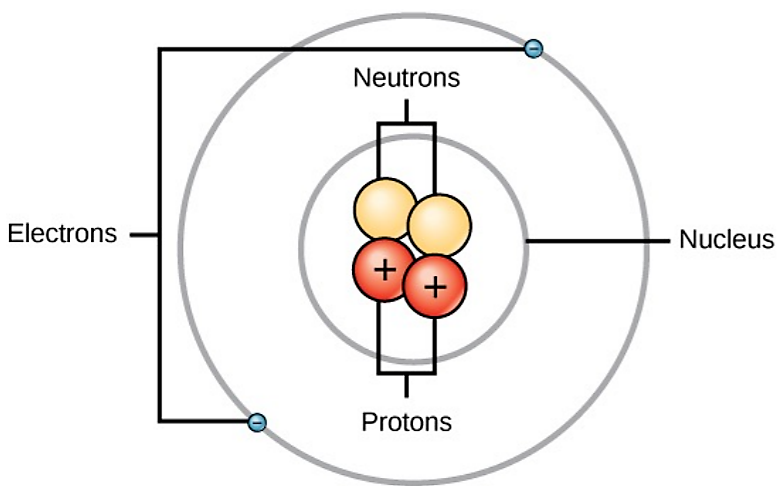
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, one gold atom has all of the properties of gold in that it is a solid metal at room temperature. A gold coin is simply a very large number of gold atoms molded into the shape of a coin and containing small amounts of other elements known as impurities. Gold atoms cannot be broken down into anything smaller while still retaining the properties of gold. An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outermost region of the atom which holds its electrons in orbit around the nucleus, as illustrated in Figure \(\PageIndex{1}\) Atoms contain protons, electrons, and neutrons, among other subatomic particles. The only exception is hydrogen (H), which is made of one proton and one electron with no neutrons.
.png?revision=1&size=bestfit&width=450&height=283)
Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) (Table \(\PageIndex{2}\)). Although similar in mass, protons and neutrons differ in their electric charge. A proton is positively charged whereas a neutron is uncharged. Therefore, the number of neutrons in an atom contributes significantly to its mass, but not to its charge.
| Charge | Mass (amu) | Location in atom | |
|---|---|---|---|
| Proton | +1 | 1 | Nucleus |
| Neutron | 0 | 1 | Nucleus |
| Electron | -1 | 0 | Orbitals |
Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. Hence, they do not contribute much to an element’s overall atomic mass. Although not significant contributors to mass, electrons do contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In uncharged, neutral atoms, the number of electrons orbiting the nucleus is equal to the number of protons inside the nucleus. In these atoms, the positive and negative charges cancel each other out, leading to an atom with no net charge. Accounting for the sizes of protons, neutrons, and electrons, most of the volume of an atom—greater than 99 percent—is, in fact, empty space. With all this empty space, one might ask why so-called solid objects do not just pass through one another. The reason they do not is that the electrons that surround all atoms are negatively charged and negative charges repel each other. When an atom gains or loses an electron, an ion is formed. Ions are charged forms of atoms. A positively charged ion, such as sodium (Na+ ), has lost one or more electrons. A negatively charged ion, such as chloride (Cl- ), has gained one or more electrons.
Molecules
Molecules are formed when two or more atoms join together through chemical bonds to form a unit of matter. Throughout your study of environmental science, you will encounter many molecules including carbon dioxide gas. Its chemical formula is CO2, indicating that this molecule is made up of one carbon atom and two oxygen atoms. Some molecules are charged due to the ions they contain. This is the case for the nitrate (NO3- ), a common source of nitrogen to plants. It contains one nitrogen atom and three oxygen atoms, and has an overall charge of negative one.
Isotopes
Isotopes are different forms of an element that have the same number of protons but a different number of neutrons. Some elements—such as carbon, potassium, and uranium—have naturally occurring isotopes. Carbon-12 contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 (six protons and six neutrons). Carbon-14 contains six protons, eight neutrons, and six electrons; its atomic mass is 14 (six protons and eight electrons). These two alternate forms of carbon are isotopes. Some isotopes may emit neutrons, protons, and electrons, and attain a more stable atomic configuration (lower level of potential energy); these are radioactive isotopes, or radioisotopes. Radioactive decay describes the energy loss that occurs when an unstable atom’s nucleus releases radiation, for example, carbon-14 losing neutrons to eventually become carbon-12.
Carbon
The basic functional unit of life is a cell and all organisms are made up of one or more cells. Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds with as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Hydrocarbons
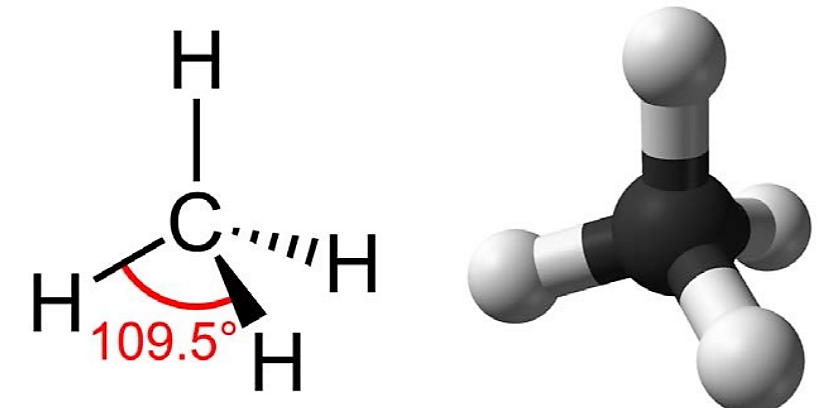
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4). We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned (oxidized). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms, as illustrated in Figure \(\PageIndex{2}\).
.png?revision=1&size=bestfit&width=548&height=267)
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.

