Polymer Chemistry: Polymerization Reactions
- Page ID
- 2914
For polymerization to take place, the monomers must be capable of being linked to two (or more) other monomer molecules2 . Most of these reactions can be placed in one of two classifications: chain-reaction polymerization and step-reaction polymerization.
Chain-Reaction Polymerization
Chain-reaction polymerization, sometimes called addition polymerization, requires an initiator to start the growth of the reaction. The largest family of polymers 3, vinyl polymers, are produced by chain polymerization reactions. A good example is the free-radical polymerization of styrene, which is initiated by a free radical (R) that reacts with styrene. The compound that is formed still is a free radical, which can react again.
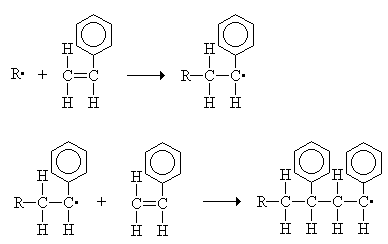
This reaction eventually leads to the formation of polystyrene, a portion of which is shown below. Polystyrene prepared by free-radical polymerization is
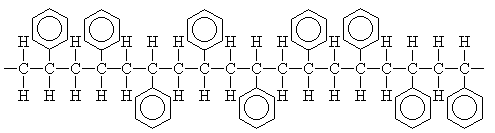
Polystyrene can be represented using a shorthand notation.
Styrene is an example of a vinyl monomer - a small molecules containing carbon-carbon double bonds. A few other important vinyl monomers are listed below4. Some of these polymers are synthesized by methods involving initiators other than free radicals - carboanions, carbocations, or coordination compounds, for instance. Vinyl polymers are addition polymers, which have the same atoms as the monomer in their repeat units.
| Monomer | Polymer | ||
|---|---|---|---|
| ethylene | polyethylene | ||
| propylene |  |
polypropylene |  |
| vinyl chloride |  |
poly(vinyl chloride) (PVC) |  |
| vinylidene chloride |  |
poly(vinylidene chloride) (Saran) |  |
| methylmethacrylate |  |
poly(methylmethacrylate) (PMMA) |  |
| Produced by hydrolysis of poly(vinyl acetate) | poly(vinyl alcohol) (PVA) |  |
|
Step-Reaction Polymerization
In a step-reaction polymerization reaction, sometimes called condensation polymerization, the polymer chains grow by reactions that occur between two molecular species. An example is the polymerization reaction involving terephthalic acid and ethylene glycol, both of which are bifunctional.
Polymer formation begins with one diacid molecule reacting with one dialcohol molecule to eliminate a water molecule and form an ester. The ester unit has an alcohol on one end and acid on the other, which are available for further reactions.
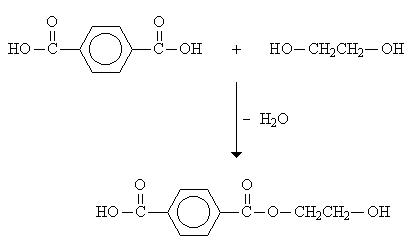
The eventual result is a polyester called poly(ethylene terephthalate) or more commonly, PET.
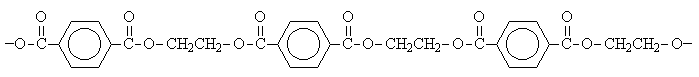
PET is the plastic in soda bottles. It can be represented with a shorthand notation.

Polyesters are condensation polymers, which contain fewer atoms within the polymer repeat unit than the reactants because of the formation of byproducts, such as H2O or NH3, during the polymerization reaction. Most synthetic fibers are condensation polymers.
A few types of condensation polymers are listed below. In the table, R and R' stand for organic groups.
| Typical Monomers | Polymer Type | ||
|---|---|---|---|
 |
polyester |  |
|
 |
polyamide (nylon) |  |
|
| polyurethane |  |
||
 |
polycarbonate |  |
|
Contributors and Attributions
David Whisnant (Wofford College). Partial support for this work was provided by the National Science Foundation's Division of Undergraduate Education through grants DUE #9950809 and DUE #9950296. Additional support was provided by the Camille and Henry Dreyfus Foundation.


