1.8: Passivation
- Page ID
- 34965
Another effect that limits the rate of corrosion is passivation. If the potential of an electrode is raised above some passivation potential, a passive product may become favourable forming a layer on the surface of the anode. In this case, the rate of corrosion can be much reduced. This is characterised by the value of log (i) peaking at a critical current density, before falling to some lower value.
In other words, the anodic arm of the Tafel plot reaches a peak and falls away to a roughly horizontal region:
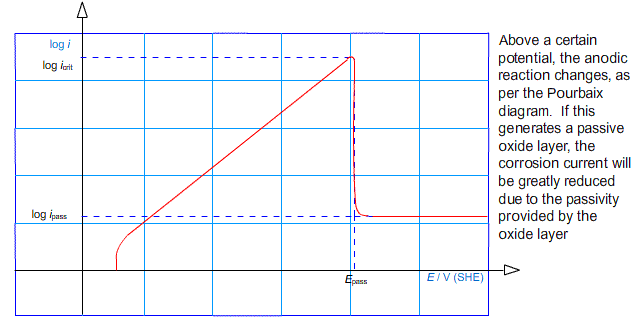
It is possible to deliberately drive the reaction into the regime in which a passive layer forms. This technique is used in the process known as anodising in which thick oxide layers are developed on aluminium components.
The Tafel plot below shows two electrodes. The cathodic branch of the electrode with the higher equilibrium potential (shown in blue) is diffusion limited. The anodic branch of the electrode with the lower equilibrium potential (shown in red) is passivated. The resulting intersection is often a good representation of real corrosion scenarios, where the metal can passivate and there is a limited oxygen supply for the cathode.


