4.5: Fuel and Cladding
- Page ID
- 8189
Choice of Fuel
There are several important factors when choosing a nuclear fuel:
- The fuel itself must be easily fissionable, preferably fissile.
- The fuel must release sufficient quantities of neutrons per neutron captured to be able to sustain a fission chain reaction. Too many neutrons produced and a runaway, supercritical, reaction would occur which would be disastrous in the case of a nuclear reactor. The ratio of neutrons produced to neutrons absorbed can, however, be adjusted through use of control rods and moderators.
- The fuel must have a sufficiently long half-life. Fissile materials, by their very nature due to their instability, are radioactive. Radioactive materials decay exponentially, and this decay is quantified by their half-life, the time it takes for half of the radioactive nuclei present to decay into a more stable form. Nuclear fuels must therefore have a sufficiently long half-life, otherwise the nuclei would decay into a useless form before fission could be induced in a controlled manner.
- Economic factors are also important. The fuels must be abundant and readily available. Uranium is the only naturally abundant fissile material and exists in an ore, called uraninite (also known as pitchblende), which is primarily uranium (IV) oxide, mined primarily in Canada, Australia and Kazakhstan. It has an isotopic composition of 99.3% of the fissionable but not fissile 238U and just 0.7% of the fissile 235U. This means that it must first be enriched, a difficult and expensive process which raises the proportion of 235U to 238U.
- No plutonium occurs naturally, except in trace amounts as a result of the natural decay of uranium. It is instead made as a by-product in nuclear reactors, and must first be extracted from use nuclear fuels before it can be used.
- Political concerns are important; heavily enriched uranium and plutonium can be used for atomic weaponry and so are not favoured. This is why there is current interested in the thorium cycle, which produces 233U. Though this can in theory be used in atomic weaponry, it is always contaminated with 232 U, which is highly dangerous because of the amount of gamma radiation it emits and making it very difficult to handle. Before the 233U could be used as a weapon, the 232U would have to be removed, which is again very difficult. This inherent proliferation resistance, and thorium’s natural abundance (3–4 x as abundant as uranium), has increased interest in it in recent years.
- The common fission fragments formed are also important, both in the short-term due to the effects they have on structural materials in the reactor and also in the long-term since some fragments have very long half-lives and so will present problems as nuclear waste since it will need to be stored for much longer periods of time. It should be noted, however, that the longer the half-life of the fission product the less dangerous it is to people. This is a somewhat counter-intuitive point that is often missed, since a longer half-life means that less of the material decays and hence gives off dangerous radiation in a given period.
Form of Fuel
Metallic uranium is not favoured as a fuel since it is dimensionally unstable under irradiation, flammable, can readily corrode in oxygen-containing atmospheres, and can produce uranium dust which has a low-temperature flash-point and which can cause serious health problems if inhaled. There is some interest in using metallic U alloys as fuel when a particularly high density of fissile or fissionable nuclides is required.
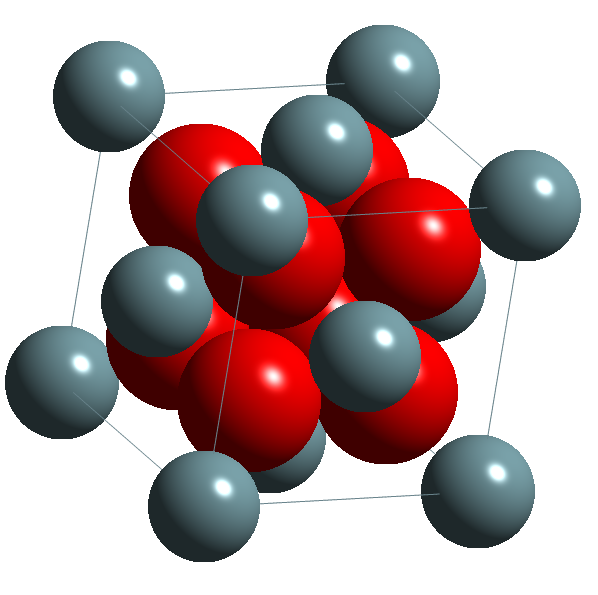
As an alternative, ceramic forms can be used, including UO2, U3O8, UC, U2C3, UN, U3Si and USi. The most common of these is uranium dioxide, which has the calcium fluorite structure shown in the image below.
Choice of Cladding
The nuclear fuel cannot be allowed to make direct contact with the coolant inside the reactor vessel, due to the potential for radioactivity to be released into the environment. Instead, cladding has to be used to surround the fuel.
Key design criteria are that the cladding should:
- be transparent to neutrons, so that it doesn’t absorb neutrons that could be used to induce further fission.
- have a high thermal conductivity, and not have a high thermal expansion coefficient.
Key problems include:
- hydrogen embrittlement due to (n, p) reactions inside cladding.
- swelling due to release of fission product gases.
Common choices for cladding material are stainless steel (in FBRs) Zircaloy (in PWRs) and, in the past, Magnox.


