1.2: Molecules
- Page ID
- 31534
When atoms are close enough to one another that the outer electrons of one atom can interact with the other atoms, then attractions can be set up between atoms, strong enough to hold them together in what is termed a chemical bond. In the simplest cases the bond arises from the sharing of two electrons between a pair of atoms, with one or more electrons provided by each of the bonded atoms. Bonds based on electron sharing are known as covalent bonds, and two or more atoms held together as a unit by covalent bonds are known as a molecule.
In molecular diagrams, a covalent, electron-sharing bond is represented by a straight line connecting the bonded atoms. In the water molecule, one atom of oxygen (O) is bonded to two hydrogen (H) atoms. One way of representing this molecule visually is as follows:
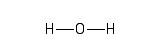
The second version acknowledges the fact that a water molecule is not linear; the two H - O bonds make an angle of 105° with one another:
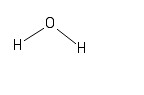
These diagrams show only the connections between atoms in the molecules. They do not show the three-dimensional geometries (or shapes) of the molecules, but you have likely seen such structures in prior chemistry courses. We will not explore those 3-D structures in detail in this course, but will instead typically use one of the two formats above, or the molecular formula approach presented next.
Molecular formulas tell how many atoms of each element are in the molecule, but provides little or no information as to how the atoms are connected. The molecular formula for hydrogen is H2; water, H2O; hydrogen sulfide, H2S; ammonia, NH3; methane, CH4 ; methyl alcohol, CH3OH or CH4O; and octane, C8H18.
The sum of the atomic weights of all the atoms in a molecule is its molecular weight. Using the atomic weights of individual atoms, we can calculate molecular weights.
The molecular weight of hydrogen, H2, is
2 X 1.0080 amu = 2.0160 amu
A water molecule, H2O , has two atoms of hydrogen and one atom of oxygen, so:
(2 X 1.0080 amu) + (15.9994 amu) = 18.0154 amu
In these examples, the atomic weights and, hence, molecular weights, are calculated to four decimal places. For our purposes, you can feel free to be slightly less precise:
If an atomic weight is very near to an integer and would round to 0 in the first decimal place, you can use that integer. Examples include carbon (12.01 --> 12.0), oxygen (15.999 --> 16.0), hydrogen (1.008 --> 1.0), nitrogen (14.007 --> 14.0), and others.
When an atomic weight would not round to an even integer but would have a non-zero value in its first decimal place, include that first decimal place after rounding. Examples include chlorine (35.45 --> 35.5), magnesium (24.31. --> 24.3), iron (55.845 --> 55.8), etc.
| Calculate the molecular weight of methanol (methyl alcohol), CH3OH. | ||||||||||||
|
Solution The molecular formula is CH3OH or CH4O. Then:
|
| What is the molecular weight of trichloroethylene (TCE), or C2HCl3? | ||||||||||||
|
Solution Since the molecular formula is C2HCl3 the molecular weight is:
|

