11.4.1.3: Lithium-Ion Batteries
- Page ID
- 84612
For most of the 20th century, research into new types of batteries continued to be stagnated. In fact, the Ni-Cd rechargeable battery (invented in 1899) was the only mass-produced type with energy density higher(by about 50%) than the lead-acid.
In 1980 an American scientist, John Goodenough, invented a battery with entirely new lithium-based chemistry. Right from the beginning it was clear that the energy density of this new type was far superior to that of all previously known kinds of rechargeable batteries. The new “lithium ion” technology was first commercialized by SONY in 1991. The lightweightness of the new power sources made possible the emergence of new generations of portable electronics (e.g., smartphones, tablets), cordless power tools, electric cars, and last but definitely not least, of industrial-scale energy-storage batteries capable of smoothing out the fluctuations in power provided by intermittent sources such as solar panels and wind turbines.
In electrochemical cells normally more than one species of ions participates in transmitting current between the electrodes across the electrolyte. For instance, in lead-acid batteries these are the negative HSO4ions and positive H+ ions. In contrast, in the family of lithium-ion batteries only a single kind is involved – namely, positive Li+ ions. Another difference is that in the lithium-ion cells the material of the electrodes is not a subject to chemical reactions. The electrodes are made of materials that can act as “sponges” for the Li ions. Just like a bathroom sponge that absorbs water or releases it when squeezed, the electrodes can absorb the ions from the electrolyte, or they can release them back.
The physio-chemical process underlying such “sponge-like” action is known as intercalation. There is a number of materials in which the constituent atoms or molecules are organized into layers, stacked on the top of each other – in such a fashion that that relatively wide “gaps” remain between the layers. Wide enough that they can accommodate small atoms of other elements. The best known “host” of such kind is graphite, one of the bulk crystalline forms of carbon (the other is diamond).
In a single layer (monolayer) in graphite the carbon atoms are arranged into a two-dimensional (2D) hexagonal lattice (often referred to as a “honeycomb lattice”). A single layer of this kind, if individually prepared, is known as graphene3. Graphite can be then thought of as a solid consisting of a large number of graphene monolayers. The three-dimensional stacking arrangement in graphite is such that at above the center of each elementary hexagon in one layer (call it A) there is an atom belonging to the next layer (call it B) – and, inversely, under the center of each hexagon in Layer B there is an atom belonging to Layer A. The arrangement of atoms in graphene and in graphite is very well illustrated by figures and animations in the two Web sites linked above, it is strongly recommended to watch them.
The distance between two carbon atom nearest neighbors in each layer is 0.2461 nanometers (nm), while the layer-layer distance is 0.3353 nm. Such “gap” is wide enough to accommodate lithium atoms – a bulk specimen of graphite can absorb one lithium atom per each 6 carbon atoms, so that the LiC6 symbol is often used for graphite fully “saturated” with intercalated Li atoms.
Graphite is used as the negative electrode (anode) in almost all sub-types of lithium-ion batteries. There is a variety of materials used for positive electrodes. One particularly interesting such compounds is cobalt dioxide CoO2 which was used in the very first lithium-ion batteries by their inventor, John Goodenough. The material also has a layered atomic structure. The “basic block” in it is an octahedron. The octahedron can be thought of as two pyramids sharing a common square base.
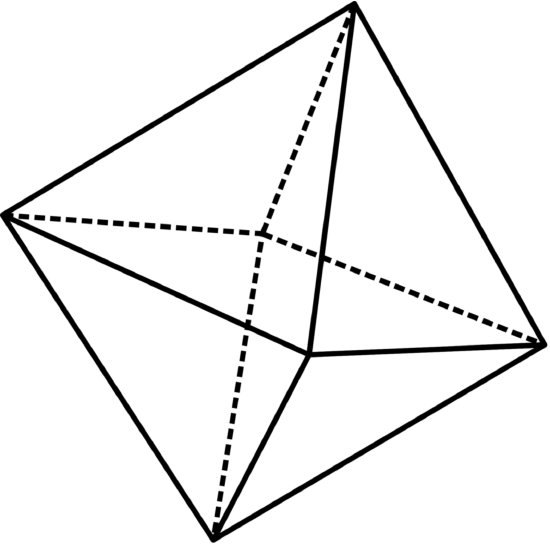
In CoO2 the basic octahedron is formed by six oxygen atoms located at its six vertices, with the cobalt atom residing in the center of the common square base. The octahedra are arranged into layers with relatively wide “gaps” between them (see the Fig. \(\PageIndex{1}\)). Therefore CoO2 can become an “intercalation host” for lithium. The gaps can accommodate one lithium atom for each cobalt atom – so, when fully intercalated, the formula of the system becomes LiCoO2 (lithium cobalt dioxide). But the intercalation process is fully reversible, CoO2 may be thought of as a “fully de-intercalated” lithium cobalt dioxide. The system may exist in any intermediate form between CoO2 and LiCoO2, and then the formula is LixCoO2, where x is a number 0 < x < 1.
The anatomy of a lithium-ion battery. The two intercalation electrodes are separated by a layer of electrolyte, which is a solution of a lithium salt (most often, it’s lithium hexafluorophosphate LiPF6) in an organic solvent. The electrons flow in or out of the electrodes through contact plates. These plates are directly connected to the cell terminals. Inserted into the electrolyte, between the two electrodes, is a separator – a thin porous flexible plate or a mesh through which ions can pass freely, but it prevents short circuits between the two electrodes (i.e., touching one another).
The arrangement of the electrodes, the contact plates, the electrolyte and the separator is shown schematically in the Fig. 11.7. The parts (a) and (b) of the figure show the flow of Li+ ions in the electrolyte, and of electrons in the external circuit when, respectively, current is drawn from the battery during normal operation, and during battery recharging. In the next Figure \(\PageIndex{2}\) the flow of Li+ ions and electrons is shown only for a normal currentdrawing operation, but here more attention has been paid to showing the atomic structure of the graphite anode and the LixCoO2 cathode.

When current is drawn from the battery the electrons flow out of the graphite anode through the external circuit (in the Fig. \(\PageIndex{2}\) (a) it contains a light bulb) and reach the cobalt oxide cathode. But charge cannot get accumulated in the electrodes! Therefore, the influx of the negative charge of electrons must be “neutralized” by the influx of the same amount of positive charge – and this charge is supplied by positive Li+ ions entering the electrode from the electrolyte.
A reverse process occurs at the same time in the anode: the outflow of electrons would charge it positively therefore, the electrode prevents the accumulation of this charge by ejecting the positive Li+ ions. As follow from the above, the current-supplying phase may last as long as there are still ions in the anode, and there is still space in the cathode for accommodating additional ones.
In the battery charging phase (Fig. \(\PageIndex{2}\) (b) all these processes are reversed. A device known as a “charger” now forces electrons to leave the cathode and enter the anode – so that the Li+ ions are now ejected from the cathode and re-enter the anode. Note that the statement electrons leave the cathode means, at the same time, that classical current flows into it – in other words, during the re-charging process the cathode and anode change their roles, the cobalt oxide electrode becomes the anode, and the graphite electrode becomes the cathode.
Note that in contrast to the lead-acid battery the electrolyte does not participate in any chemical reactions – it acts only as a “passive” medium allowing the passage of Li+ ions between the electrodes.
In Fig. \(\PageIndex{3}\) there is another graph showing the “anatomy” of a Li-ion cell, with more attention paid to the atomic structures of the electrodes that allows them to serve as “intercalation hosts” for the Li+ ions: namely, the hexagonal graphene-like atomic layers in the graphite anode, and the layers built of elementary CoO6 tetrahedra in the cobalt oxide cathode.
In fact, the anatomy of real commercially available Li-ion batteries is not very similar to what is shown in the Fig. \(\PageIndex{3}\). The most common battery versions are cylindrical in shape. A special five-digit designation code has been created for such Li-ion cells: the first two digits are the cylinder diameter in millimeters, and the other three digits indicate the cylinder height in tenths of a millimeter. Accordingly, the most popular cell (i.e., being produced in the largest number and used, among others, in Tesla cars) is the “18650”, slightly larger than the AA battery (whose “code name”, if the same rule was used, would be 14500).

The electrodes in such batteries are made of graphite and CoO2 ground to powders consisting of microcrystals and mixed with a plastic-like compound which makes it possible to prepare them in the form of long thin “ribbons”, or stripes. The contact plates are also replaced by strips made of aluminum (Al) an copper (Cu) foil. The remaining two elements, the electrolyte and the separator, can be prepared jointly in the form of a stripe of fabric (e.g., made of glass fibers) soaked with the electrolyte. Then, the stripes are stacked on top of each other in the following order: Al foil stripe, the CoO2 cathode stripe, separator with electrolyte stripe, graphite anode stripe, and the Cu foil stripe. The idea is to roll the stacked stripes into a tight “scroll”. However, in such a scroll the Al and Cu foils would touch one another; therefore, one extra stripe made of insulating foil is put at the very bottom of the stack.

After being rolled into a tight “scroll”, the whole assembly is put into a cylindrical capsule, most often made of steel – with the terminals at the two cylinder bases. But “rolling” is not the only possible way of processing the stripe stack – another method used by some manufacturers is “accordion folding” at which one gets a “sandwich” that can be put into a flat capsule. Such li-ion batteries are commonly referred to as “prismatic”. Some electric vehicle (EV) manufacturers (e.g., Tesla) use cylindrical cells because when thousands of them are assembled into large batteries there is space left for easy coolant circulation (during a rapid charge operation the cells must be intensively cooled). Other (e.g., Nissan Leaf or BMW) prefer the prismatic type because they offer a better stored energy per unit volume ratio. Prismatic cells are also the obvious choice for portable electronics devices, such as, e.g., cell phones or tablets.
_______________________________________________________________
3It is not trivial to prepare large specimens such two-dimensional layers; Andre Geim and Konstantin Novoselov who first mastered the technique in 2004, were awarded a 2010 Nobel Prize in physics for that

