11.4.5.3: The recovery of energy stored in the form of hydrogen
- Page ID
- 84625
Hydrogen \(\to\) Heat: the easiest method is simply to burn hydrogen for heat. Just like we do it with natural gas we use it to heat homes, in kitchen stoves, in hot water heaters. Hydrogen can replace natural gas – and it does not have to happen right away – one can start with blending, say, 20
But further increasing the hydrogen content of the gas will not be quite easy, for several reasons. The first is the low heat of hydrogen combustion yes, low. How comes? After all, methane has a heat of combustion of 55.1 MJ/kg, and hydrogen up to 141.6.1 MJ / kg, 2.57 times higher! Right! But let’s not forget that the gas bill is not for kilograms, but for cubic feet.
With gases it is so that at a certain temperature and pressure, one mole of gas always occupies the same volume. A mole of hydrogen is 2 grams, and a mole of methane 16 grams eight times more. Thus, one cubic foot of methane has a heat of combustion of 8 / 2.57 = 3.1 times greater than that of hydrogen. A more professional way is to say: hydrogen’s volumetric heat of combustion is 3.1 times lower than that of methane.
What does it mean? To provide the users with the same amount of energy as with methane, you will need to supply them with three times the volume of hydrogen. Existing pipelines for something like this are not prepared therefore, complete transition from natural gas to hydrogen will require changing pipes in gas pipelines with new ones of a larger diameter. Similarly, home heating stoves, kitchen stoves, hot water heaters and even gas grills will require far-reaching alterations. It will be a huge and very expensive work that will take years. But the alternative is to do nothing and accept the increasing concentration of CO2 in the atmosphere. Moneywise, the cost will be less, but the overall price the society will have to pay not necessarily in money may turn out to be higher.
Hydrogen → Electric power. The energy stored in hydrogen can also be recovered in the form of electricity. We will present two types of devices that enable such conversion.
The first of these are fuel cells. They can be thought of as devices in which water electrolysis “takes place in reverse”. An electrolyzer takes electric energy as an input, and delivers oxygen and hydrogen as the output products. In a fuel cell, hydrogen and oxygen are the input, and electric energy is the output product (with water vapor as a byproduct).
The oldest “ancestor” of modern fuel cells was invented as early as in 1838, but the first usable practical one was the 5 kW “alkaline” cell developed by a British engineer F.T. Bacon in 1932. More intensive research on fuel cell technology began around 1950. As a result, a whole spectrum of new types was created (some of them may use other fuels than hydrogen, e.g., methanol, but here we limit our attention only to those using hydrogen).
All hydrogen fuel cells have a similar structure: they feature two electrodes, usually made of porous metals (so that gases can penetrate them), separated by a layer of electrolyte. Hydrogen is fed to one electrode, oxygen to the other. Most often, instead of pure oxygen, air is used because nitrogen and other air components do not interfere with the cell action.
In a PEM-type fuel cell (PEM = Proton Exchange Membrane a.k.a. Polymer Electrolyte Membrane), the electrolyte is a made of a substance that enables a flow of H+ ions, i.e., of protons. Hydrogen gas is fed to the anode, in which, due to the presence of platinum catalyst, H2 molecules are ionized by giving away two electrons. These electrons travel through the external circuit to the cathode, where they – again aided by a catalyst – join oxygen atoms to form O2− ions. At the same time, the H+ ions flow across the PEM membrane to the cathode, where they recombine with the O2− ions, forming H2O molecules. In short, in the cell oxygen and hydrogen combine, creating water, and causing a flow of electric current through an external circuit. The PEM cells typically operate at temperatures of 80 100◦C.
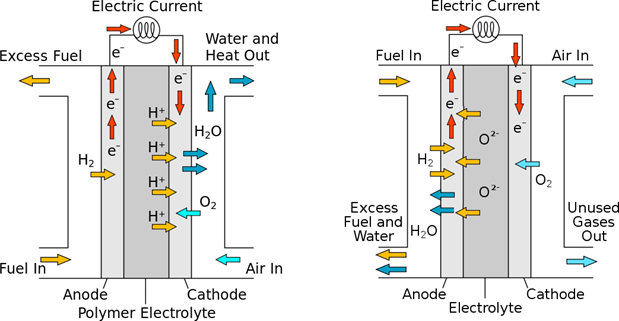
In solid oxide fuel cells (SOFC), in contrast, it’s the O2 ions that travel from the cathode to the anode, through the electrolyte made of metal oxide ceramics. Such ceramics acquires ionic conductivity only at high temperatures, so that the SOFC cells operate in the temperature range between 700◦C and 1000◦C.
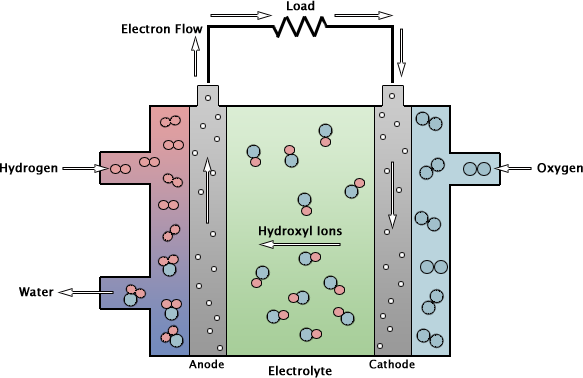
Yet another type of ionic conductivity is used in alkaline fuel cells. Invented in 1932, they have the simplest design but despite the lack of sophistication, they, as the only fuel cells to date, traveled to the Moon aboard the “Apollo” spacecrafts. The electrolyte in these cells is a porous substance saturated with an aqueous solution of potassium hydroxide KOH. In this solution, KOH dissociates into K+ and OH− ions – so that here ionic conductivity is a natural phenomenon. In the cathode, into which electrons arrive through an external circuit, water, oxygen and electrons react to form hydroxyl ions OH−. Next, these ions flow through the electrolyte to the anode, where there are H+ ions awaiting them (they are created from hydrogen which, aided by a catalyst, donates electrons to the external circuit). As the result of H+ and OH− recombination water, is formed.
Phosphoric acid fuel cells (PAFC) are highly reliable sources of electric current. Their scheme is essentially the same as that of the PEM cell, but they use as the electrolyte a porous matrix soaked with phosphoric acid H3PO4. They operate at 150 200◦C.

An interesting high-temperature fuel cell type is the Molten Carbonate Fuel Cell. It uses a mixture of carbonate salts as the electrolyte and an unusual ionic conductivity mechanism. Namely, the gas delivered to its cathode is a mixture of oxygen O2 with carbon dioxide CO2. There the gases react with incoming electrons to form CO2− ions that travel across the molten electrolyte. At the anode, they react with H+ ions, forming molecules of H2O and CO2. The latter is recycled to the cathode.
Essential parameters of the above four types, such as the electric power they deliver and the efficiency of they offer are given in the Table in Fig. 11.22. Note that fuel cells can be expected to convert into electricity up to 60% of the energy contained in the stored hydrogen.
Why only that much? Because in this case it’s physics that comes to the fore! As we remember, in the case of water electrolysis, the energy contained in the hydrogen produced1 may be greater than that brought in by the electricity used, because, as has been mentioned before, a certain slice of energy is added “by the environment”. In other words, it’s Mother Nature who adds some “free extra bonus” to the energy of electrolytically produced hydrogen. But if you want to use this hydrogen to energize a fuel cell, then Mother Nature will order you to give her that “bonus” back! More seriously it’s physics that rules that some part of the chemical energy the hydrogen and oxygen bring together into the system must be converted to heat, not to electricity. Note that here there is a certain analogy with heat engines, in which as we have already discussed in Chapter 2 only part of the input energy can be converted to mechanical work, and the rest must be discharged as “waste heat” (but this is only a superficial analogy, because the detailed theory of heat engines and the famous Carnot Equation does not apply to fuel cells!). A closer analysis of the thermodynamic relationships in the fuel cell (which also apply to water electrolysis) is presented in this Hyperphysics website.
The efficiency of fuel cells may, however, be regarded as higher if we do not consider the heat emitted in them as “ waste heat ”, but as a valuable commodity. Everybody who lives in cooler climate appreciates the value of heat, especially in the wintertime!
In view of this, it is conceivable to employ a larger fuel cell installation as a source of electricity and heat for a number of households. Such an idea is not anything new – it is known as cogeneration and it has been implemented in many locations all over the world, serving communities as large as entire city districts, as well as villages with only a hundred households or so. The existing cogeneration systems are usually build around conventional fossilfuel thermal power plants (and sometimes geothermal plants).
Cogeneration using fuel cells has already found some applications – for example, it provides heating in electric cars and buses powered by such cells. However, this is a very modest “niche”. Stationary installations could certainly serve household communities. An average household in the US uses an average of 1400 W of heating power – as in summertime it’s not needed, so in wintertime it is probably of the order of 3000 W. As follows from the table in Fig. 11.22, the electric power provided by an installation using the SOFC technology may be a high as 2 MW. Suppose that the co-generated heat power is only 1 MW – it still will be enough for over 300 households in the wintertime.
It may sound as an attractive option for some rural communities and townships – but it seems that there is not much interest for such an idea. Why? Probably because, as for now, all hydrogen produced worldwide is used by a few industrial branches and is not so cheap. Well, perhaps when
the “hydrogen economy”, now in its infancy, will grow in strength and begin to flex it muscles, such installations will begin to emerge...
Gas turbines fueled by hydrogen. Fuel cells offer a direct method of converting the energy contained in hydrogen into electricity – an alternative way, less direct but of similar efficiency is to use a gas turbines. Such turbines, widely used by utility companies, now use natural gas as fuel. The latest models have the highest efficiency of all heat engines, even above 60%.
Manufacturers of these turbines already are aware that governments, as part of decarbonization policy, will impose increasingly stringent restrictions on the use of methane fuel. So they have the choice of either stopping the production of turbines or reworking them so that they can use pure hydrogen instead of methane. They clearly opt for the latter. Intensive research is underway on how to manage this type of conversion. For now, they are testing the use of fuel with a relatively small amount of hydrogen, 10-20%. Turbines tolerate this well, but further increasing the concentration of hydrogen up to 100% will already require major alterations of the fuel system. We will not describe the current status of this research here, but we provide links to four recent reports discussing these issues: Report number one, Report number two, Report number three, and Report number four.
In summary when the “hydrogen economy” reaches its maturity and gathers strength, it may become a stage of competitive struggle between hydrogen fuel cells and hydrogen gas turbines. We have to wait to see...
______________________________________________________________
1If we say that “hydrogen contains energy”, it should be kept in mind that this is only a “figure of speach”. The actual carrier of the energy stored is a pair of gases, hydrogen and oxygen. But we don’t store the latter because for recovering the energy we can simply use the oxygen which is abundant in the air.

