3.3.9: Light Emitting Diode
- Page ID
- 89957
Let's talk about the recombining electrons for a minute. When the electron falls down from the conduction band and fills in a hole in the valence band, there is an obvious loss of energy. The question is; where does that energy go? In silicon, the answer is not very interesting. Silicon is what is known as an indirect band-gap material. What this means is that as an electron goes from the bottom of the conduction band to the top of the valence band, it must also undergo a significant change in momentum. This all comes about from the details of the band structure for the material, which we will not concern ourselves with here. As we all know, whenever something changes state, we must still conserve not only energy, but also momentum. In the case of an electron going from the conduction band to the valence band in silicon, both of these things can only be conserved if the transition also creates a quantized set of lattice vibrations, called phonons, or "heat". Phonons posses both energy and momentum, and their creation upon the recombination of an electron and hole allows for complete conservation of both energy and momentum. All of the energy which the electron gives up in going from the conduction band to the valence band (1.1 eV) ends up in phonons, which is another way of saying that the electron heats up the crystal.
In some other semiconductors, something else occurs. In a class of materials called direct band-gap semiconductors, the transition from conduction band to valence band involves essentially no change in momentum. Photons, it turns out, possess a fair amount of energy (several eV per photon in some cases) but they have very little momentum associated with them. Thus, for a direct band gap material, the excess energy of the electron-hole recombination can either be taken away as heat, or more likely, as a photon of light. This radiative transition then conserves energy and momentum by giving off light whenever an electron and hole recombine. This gives rise to (for us) a new type of device, the light emitting diode (LED). Emission of a photon in an LED is shown schematically in Figure \(\PageIndex{1}\).
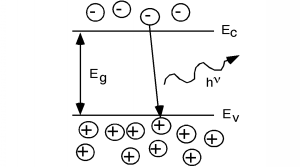
It was Planck who postulated that the energy of a photon was related to its frequency by a constant, which was later named after him. If the frequency of oscillation is given by the Greek letter "nu" \((\nu)\), then the energy of the photon is just \(h \nu\) where \(h\) is Planck's constant, which has a value of \(4.14 \times 10^{-15} \mathrm{~eV} \cdot \mathrm{seconds}\). \[E = h \nu \nonumber \]
When we talk about light it is conventional to specify its wavelength, \(\lambda\), instead of its frequency. Visible light has a wavelength on the order of nanometers (red is about 600 nm, green about 500 nm and blue is in the 450 nm region.) A handy "rule of thumb" can be derived from the fact that \(\lambda = \frac{c}{\nu}\), where \(c\) is the speed of light. Since \(c = 3 \times 10^{8} \ \frac{\mathrm{m}}{\mathrm{sec}}\) or \(c = 3 \times 10^{17} \ \frac{\mathrm{nm}}{\mathrm{sec}}\), \[\begin{array}{l} \lambda (\mathrm{nm}) &= \frac{hc}{E (\mathrm{eV})} \\ &= \frac{1242}{E (\mathrm{eV})} \end{array} \nonumber \]
Thus, a semiconductor with a \(2 \mathrm{~eV}\) band-gap should give off light at about \(620 \mathrm{~nm}\) (in the range of red light). A \(3 \mathrm{~eV}\) band-gap material would emit at \(414 \mathrm{~nm}\), in the violet. The human eye, of course, is not equally responsive to all colors. We show this in Figure \(\PageIndex{2}\), where we have also included the materials which are used for important light emitting diodes (LEDs) for each of the different spectral regions.
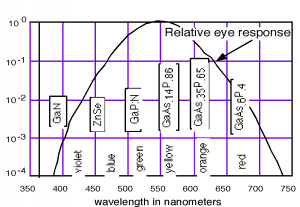
As you no doubt notice, a number of the important LEDs are based on the \(\mathrm{GaAsP}\) system. \(\mathrm{GaAs}\) is a direct band-gap semiconductor with a band gap of \(1.42 \mathrm{~eV}\) (in the infrared). \(\mathrm{GaP}\) is an indirect band-gap material with a band gap of \(2.26 \mathrm{~eV}\) (550 nm, or green). Both \(\mathrm{As}\) and \(\mathrm{P}\) are group V elements. (Hence the nomenclature of the materials as III-V compound semiconductors.) We can replace some of the \(\mathrm{As}\) with \(\mathrm{P}\) in \mathrm{GaAs}\) and make a mixed compound semiconductor \(\mathrm{Ga As}_{1-x} \mathrm{P}_{x}\). When the mole fraction of phosphorous is less than about \(0.45\) the band gap is direct, and so we can "engineer" the desired color of LED that we want by simply growing a crystal with the proper phosphorus concentration!
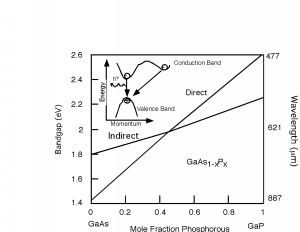
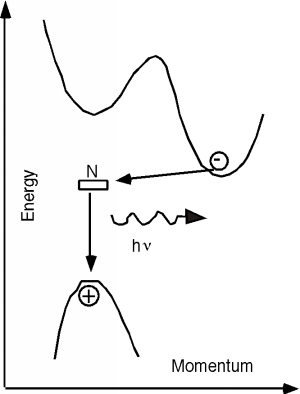
The properties of the GaAsP system are shown in Figure \(\PageIndex{3}\). It turns out that for this system, there are actually two different band gaps, as shown in the inset of Figure \(\PageIndex{3}\). One is a direct gap (no change in momentum) and the other is indirect. In \(\mathrm{GaAs}\), the direct gap has lower energy than the indirect one (like in the inset) and so the transition is a radiative one. As we start adding phosphorous to the system, both the direct and indirect band gaps increase in energy. However, the direct gap energy increases faster with phosphorous fraction than does the indirect one. At a mole fraction \(x\) of about \(0.45\), the gap energies cross over and the material goes from being a direct gap semiconductor to an indirect gap semiconductor. At \(x=0.35\) the band gap is about \(1.97 \mathrm{~eV}\) (630 nm), and so we would only expect to get light up to the red using the \(\mathrm{GaAsP}\) system for making LEDs. Fortunately, people discovered that you could add an impurity (nitrogen) to the \(\mathrm{GaAsP}\) system, which introduced a new level in the system. An electron could go from the indirect conduction band (for a mixture with a mole fraction greater than \(0.45\)) to the nitrogen site, changing its momentum, but not its energy. It could then make a direct transition to the valence band, and light with colors all the way to the green became possible. The use of a nitrogen recombination center is depicted in Figure \(\PageIndex{4}\).
If we want colors with wavelengths shorter than the green, we must abandon the \(\mathrm{GaAsP}\) system and look for more suitable materials. A compound semiconductor made from the II-VI elements \(\mathrm{Zn}\) and \(\mathrm{Se}\) make up one promising system, and several research groups have successfully made blue and blue-green LEDs from \(\mathrm{ZnSe}\). \(\mathrm{SiC}\) is another (weak) blue emitter which is commercially available on the market.
Semiconductors making visible deep blue have not been found, so building a true, quality 3-color system with a blue, green and red led is impossible. In the 1990's researchers in Japan worked out how to make GaN semiconductor crystals. GaN has a very large direct bandgap, light produced by GaN is in the UV region, invisible (and dangerous). This is similar to light made in florescent lights (both the long-tube type and the curled-up squiggly compact lights) by exciting mercury atoms. Florescent tubes are coated on the inside with material which absorbs the UV light and re-emits it at longer wavelengths. The same process is used for LEDs made with GaN.
As industry learned how to make GaN LEDs, the price dropped rapidly. The first (but short lived) product using GaN light emitters was the Blu-Ray disc, able to store 20GB or more on a single disc, compared to 4GB for DVDs and 0.7GB for the original CD. By about 2015 GaN-based white light bulbs became competitive with florescent bulbs and took over the lighting industry.


