A single polymer chain can exist in any one of its possible conformations, from a tight coil to a straight chain. The probability of it having a particular end-to-end distance increases with the number of possible conformations that would achieve that size. There is only one possible conformation that will produce a straight chain, but as the molecule becomes more coiled the number of possibilities increases. A polymer chain will therefore tend to coil up to some extent.
The expected end-to-end distance of a chain can be estimated using a model in which a molecule is considered as being made up of a large number n of segments. Each segment is rigid, but is freely jointed at both ends, so that it can make any angle with the next segment. A model ‘molecule’ can then be built by adding each of the successive segments at a random angle, a procedure called a random walk.
How does the random walk model compare to reality?
- By the nature of a random walk, a model molecule may overlap itself. Real polymers have a finite volume, so a molecule cannot ‘crash into’ itself or other chains.
- The random walk model does not take account of any complicating forces, such as the interaction of electrons in bulky side groups, which tend to inhibit bond rotation.
- Atoms in the polymer backbone are not freely jointed.
- As a result of these simplifications, performing a random walk where each segment is a single C-C bond gives an underestimate of the end-to-end distance, real polymer chains are stiffer than predicted by the model.
- To take account of this, random walk segments are modelled as being several C-C bonds in length.We can then use a quantity called the Kuhn length, l, to represent the average length assigned to a model segment.
- The Kuhn length varies for different polymers: it is longer for a stiffer molecule.
- To illustrate this, here are some example Kuhn lengths (expressed as a multiple of the length of a C-C bond).
| Polymer |
Kuhn length / C-C bond lengths |
Notes |
| Poly(ethene) |
3.5 |
PE is very flexible (due to low torsional barriers) |
| Poly(styrene) |
5 |
PS has large side-groups which inhibit flexibility |
| DNA |
300 |
DNA is very stiff due to its double helix structure |
Calculating the root mean square end-to-end distance of a random walk ‘molecule’
In two dimensions, we can estimate the distance from end to end of a molecule modelled by a random walk, given the Kuhn length and the number of segments. Each segment is represented by a vector, 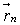
You can now test this model using the simulation below.


