9.7: Problems
- Page ID
- 18997
9.1. A 50 liter solution contains \(8 \cdot 10^{19}\) H\(^+\) ions. Calculate the pH. Is this solution acidic or basic?
9.2. A bottle contains 3 liters of a chemical solution with a pH of 8.
(a) Does the bottle contain an acid or a base?
(b) Approximately how many H\(^+\) ions are in the bottle?
(c) Would a 3 liter bottle with a pH of 9 contain more or less ions of H\(^+\) than the bottle with a pH of 8?
(d) How many times as many/few H\(^+\) ions are in the bottle with solution of pH 8 than in the bottle with solution of pH 9?
9.3. Consider a battery with a lithium electrode and a silver chloride (AgCl) electrode. Assume the following chemical reactions occur in the battery, and the redox potential for each reaction is shown.
\(\text{AgCl} + e^- \rightarrow \text{Ag} + \text{Cl}^-\) \(V_{rp} = 0.22 V\)
\(\text{Li} \rightarrow \text{Li}^+ + e^-\) \(V_{rp} = 3.04 V\)
(a) Which reaction is likely to occur at the cathode, and which reaction is likely to occur at the anode? Justify your answer.
(b) What is the overall theoretical cell voltage?
(c) If the battery is connected to a \(1 k\Omega\) load, approximately what is the power delivered to that load?
9.4. Suppose the chemical reactions and corresponding redox potentials in a battery are given by [137]:
\(\text{Li} \rightarrow \text{Li}^+ + e^-\) \(V_{rp} = 3.04 V\)
\(\text{S} + 2e^- \rightarrow \text{S}^{2-}\) \(V_{rp} = -0.57 V\)
(a) Find the overall theoretical specific capacity of the battery in \(\frac{C}{g}\).
(b) Find the overall theoretical specific energy of the battery in \(\frac{J}{g}\).
(c) Which material, lithium or sulfur, gets oxidized, and which material gets reduced?
9.5. A battery has specific capacity 252 \(\frac{C}{g}\) and mass of 50 g. Its overall density is 2.245 \(\frac{g}{m^3}\).
(a) Find the specific capacity in \(\frac{mA \cdot h}{g}\).
(b) Find the capacity in \(mA \cdot h\).
(c) Find the charge density in \(\frac{mA \cdot h}{m^3}\).
9.6. A battery has a specific capacity of 55 \(\frac{mA \cdot h}{g}\) and a nominal voltage of 2.4 V. The battery has a mass of 165 g. Find the energy stored in the battery in J.
9.7. A battery has a sulfur cathode where the reaction \(\text{S} + 2e^- \rightarrow \text{S}^{2-}\) occurs. The anode is made from a mystery material, \(X\), and at the anode, the reaction \(X \rightarrow X^{2+} + 2e^-\) occurs. The theoretical specific capacity of the sulfur reaction is 1.76 \(\frac{A \cdot h}{g}\) and the theoretical specific capacity of material X is 0.819 \(\frac{A \cdot h}{g}\). The theoretical specific capacity of the materials combined is 0.559 \(\frac{A \cdot h}{g}\). What is material \(X\), and what is \(V_{rp}\), the redox potential of the battery? (Hint: Use a periodic table and a list of redox potentials.)
9.8. What is the difference between each of the items in the pairs below?
- A battery and a fuel cell
- A primary battery and a secondary battery
- Redox potential and chemical potential
9.9. Consider the polymer electrolyte membrane fuel cell shown below. The reactions at the electrodes are:
\(\text{H}_2 \rightarrow 2\text{H}^+ + 2e^-\)
\(4e^- + 4\text{H}^+ + \text{O}_2 \rightarrow 2\text{H}_2\text{O}\)
Match the label in the picture to the component name.
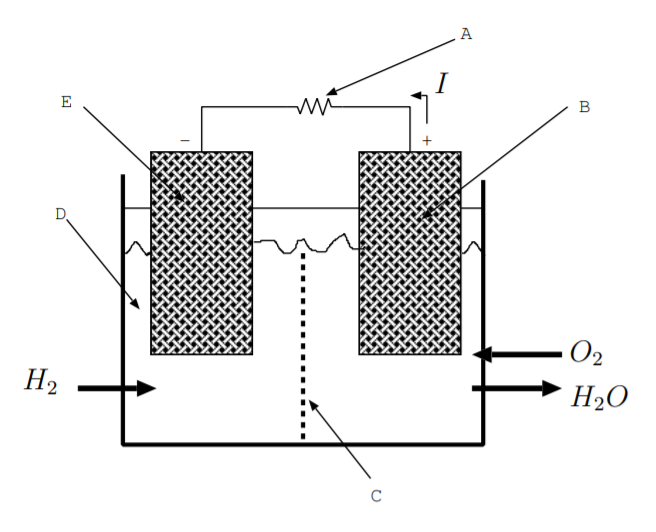
| Component name | Label |
|---|---|
| 1. Anode | |
| 2. Cathode | |
| 3. Electrolyte | |
| 4. Load | |
| 5. Polymer electrolyte membrane |
9.10. Match the name of the fuel cell components to a material used to make that component.
| Fuel cell component name | Material |
|---|---|
| 1. Anode | A. Platinum |
| 2. Byproduct (waste produced) | B. Carbon (solid, but porous) |
| 3. Catalyst | C. Water |
| 4. Electrolyte | D. Sulfuric Acid |
| 5. Fuel | E. liquid Hydrogen |


