4.2: Biomass
- Page ID
- 48561
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)4.2 Biomass
There are four types of biomass resources that can be utilized: 1) agricultural residues, 2) energy crops, 3) forestry residues, and 4) processing wastes. Examples of different sources are listed below:
Agricultural Residues:
- Corn stover
- Wheat straw
- Rice straw
- Soybean stalk
Energy Crops:
- Switch grass
- Sweet sorghum
- Sugar canes
- Algae
- Cattail
- Duckweed
Forestry Residues:
- Saw dust
- Woody chips
Processing wastes:
- Food processing wastes
- Animal wastes
- Municipal solid wastes
As already mentioned, most biomass is at least partially composed of three components: cellulose, hemicellulose, and lignin. Figure 4.2a shows a diagram of lignocellulose, and Figure 4.2b shows the biomass broken down into the three parts. There will be significantly more discussion on biomass composition in future lessons. Cellulose is a crystalline polymer of ring molecules (6 carbons) with OH and COOH groups (in Figure 4.2a, cellulose is the straight green lines; in Figure 4.2b, the green molecule). Hemicellulose is similar, but has ring molecules with 5 and 6 carbons, and is amorphous in structures, as depicted in Figure 4.2a by the black squiggly line; Figure 4.2b shows how it is around the cellulose and more detail of the molecular structure. Lignin is the material that holds it all together and is the light blue line in Figure 4.2a; it is in red in 4.2b.


How To Determine Properties of Biomass
There are four common ways to measure the properties of any carbon product, which will also be used for biomass: 1) proximate analysis, 2) ultimate analysis, 3) heat of combustion, and 4) ash analysis.
Proximate analysis
Proximate analysis is a broad measurement to determine the moisture content (M), volatile matter content (VM), fixed carbon content (FC), and the ash content. These are all done on a mass basis, typically, and are done in what is called a proximate analyzer – the analyzer just measures the mass loss at certain temperatures. Moisture is driven off at ~105-110°C (just above the boiling point of water); it represents physically bound water only. Volatile matter is driven off in an inert atmosphere at 950°C, using a slow heating rate. The ash content is determined by taking the remaining material (after VM loss) and burning it at above 700°C in oxygen. The fixed carbon is then determined by difference: FC = 1 – M – Ash – VM.
The following is an example of proximate analysis of lignin, which is part of wood and/or grasses, primarily:
- Moisture (wt%): 5.34
- Ash (wt%): 14.05
- Volatile Matter (wt%): 60.86
- FC=100−M(%)−A(%)−VM(%)FC=100−M%−A%−VM%
- FC=100−5.34−14.05−60.86=19.75FC=100−5.34−14.05−60.86=19.75
Sometimes the moisture content will be removed from the VM and ash contents, on a dry basis:
- FC=100−M(%)−A(%dry)−VM(%dry)FC=100−M%−A%dry−VM%dry
- FC=100−14.05−60.86=25.09FC=100−14.05−60.86=25.09
Ultimate analysis
Ultimate analysis is more specific in that it analyzes the elemental composition of the organic portion of materials. The compositions of carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O) are determined on a mass percent basis, and can be converted to an atomic basis. In some cases, chlorine (Cl) will also be analyzed. There are instruments that are designed to measure only the C H N mass percent and then another to measure S percent; the instrument combusts the material and measures the products of combustion. The following is an example problem for determining the molecular atomic composition of biomass when being provided with an ultimate analysis. Oxygen is usually determined by difference. Water can skew the hydrogen results and must be accounted for.
Your Turn
Problem 1:
The ultimate analysis shows that the C, H, O, N and S contents of a biomass material are 51.9%, 5.5%, 41.5%, 0.8% and 0.3% on a dry basis. What is the chemical formula of this biomass? How many kilograms of air are required to completely combust 1 kg of this biomass? The results are shown below.
The following examples are of the calculation of Problem 1, the chemical formula of biomass, when given mass percent on a dry basis. If you know the elemental mass percent of the sample, you can divide by the molecular weight to determine the atomic value of each element. The values in the table are then divided by the atomic number of carbon to normalize the molecule. So, for every carbon, you have 1.26 atoms of hydrogen, 0.6 atoms of oxygen, etc.
Table 4.1: Problem 1 Calculations
| Mass%(1/MW)=x | Values |
|---|---|
| C=51.9 (\(\frac{1}{12.011}\)) | (\(\frac{4.32}{4.32}\))=1 |
| H=5.5 (\(\frac{1}{1.0079}\)) | (\(\frac{5.46}{4.32}\))=1.260 |
| O=41.05 (\(\frac{1}{15.9994}\)) | (\(\frac{2.59}{4.32}\))=0.600 |
| N=0.8 (\(\frac{1}{14.0067}\)) | (\(\frac{0.06}{4.32}\))=0.013 |
| S=0.3 (\(\frac{1}{32.06}\)) | (\(\frac{0.01}{4.32}\))=0.002 |
Heat of combustion
The heat of combustion can be measured directly using a bomb calorimeter. This instrument is used to measure the calorific value per mass (calorie/gram or Btu/lb). It can also be estimated using different formulas that calculate it based on either ultimate or proximate analysis. A common type of calorimeter is the isoperibol calorimeter, which will contain the heat inside the jacket but will accommodate the change in temperature of the water in the bucket; see Figure 4.3 for a schematic. A sample is placed in a crucible that is put inside of a reactor with high-pressure oxygen. The sample is connected to a fuse and electrical leads that will ignite the sample, all contained within the reactor (sometimes called a bomb calorimeter). The water temperature in the bucket is measured before and after ignition, and with all the other parts calibrated, the specific heat of water and the change in temperature are used to determine the heat of combustion.
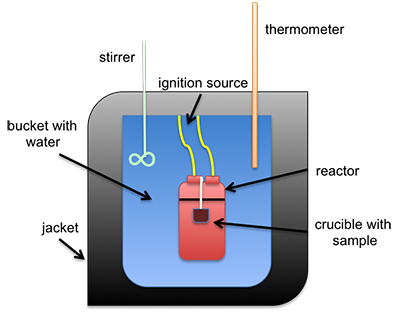
Figure 4.3: Schematic of isoperibol calorimeter.
- Click Here for a text alternative to the above image
-
This is schematic of an isoperibol calorimeter. There is a crucible containing a sample. Both sit inside a reactor. The reactor itself is in a bucket with water that has an insulating jacket on the outside, including the top. Sticking into the water is a stirrer and a thermometer. The reaction is started by an ignition source connected to the reactor.
Credit: © Penn State University, is licensed under CC BY-NC-SA 4.0
The heating value is determined in a bomb calorimeter. Heating values are reported on both wet and dry fuel bases. For the high heating value (HHV), the value can be determined by normalizing out the moisture in a liquid form. For the low heating value (LHV), a portion of the heat of combustion is used to evaporate the moisture.
Ash analysis
The minerals in the material, once combusted, turn to ash. The ash can be analyzed for specific compounds that will contain oxygen, such as CaO, K2O, Na2O, MgO, SiO2, Fe2O3, P2O5, SO3, and Cl. The original minerals can also be measured. Once the mineral or ash is isolated, it often must be dissolved in various acids and then analyzed. There is other instrumentation available, but the analysis is quite complicated and not often done.
Bulk density is also determined for biomass as a property. It is typically determined by measuring the weight of material per unit volume. It is usually determined on a dry weight basis (moisture free) or on an as-received basis with moisture content available. For biomass, the low values (grain straws and shavings) are 150-200 kg/m3 (0.15-0.20 g/cm3), and high values (solid wood) are 600-900 kg/m3 (0.60-0.90 g/cm3). The heating value and bulk density are used to determine the energy density. Figure 4.4 shows a comparison of various biomass sources to fossil fuel sources on an energy density mass basis.
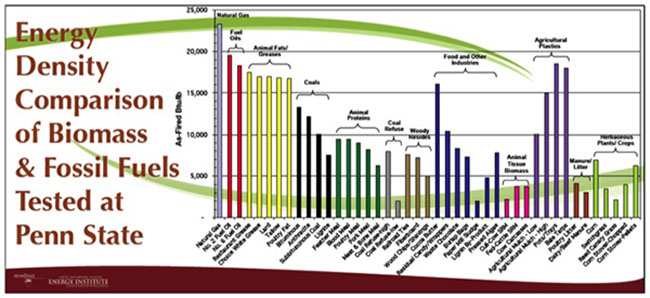
Credit: Bruce Miller, Sr. Research Associate, EMS Energy Institute, PSU
Many of the fuel characteristics we have been discussing need to be known for proper use of biomass in combustion, gasification, and other reaction chemistry.


