9.1: Humidity
- Page ID
- 47205
Air is a mixture of several gases, including nitrogen, oxygen, and water vapor.
- The total air pressure exerted by a volume of air in a given container on that container is the sum of the individual (partial) pressures of these gases.
- The vapor pressure is the individual or partial pressure of the water vapor.
The warmer the air is, the more moisture it can hold. So its moisture holding capacity changes with temperature.
A Psychometric chart, shown in Figure 9.1.1, represents the moisture content of air at various temperatures. This chart shows that as the air temperature increases, the amount of moisture that can be held in dry air also increases.
- Dry bulb temperature: Temperature with no moisture in the air; known as “absolute air".
- Dew point Temperature: Complete saturation – the maximum amount of water vapor that air can hold. Note that water vapor can carry more heat than air.
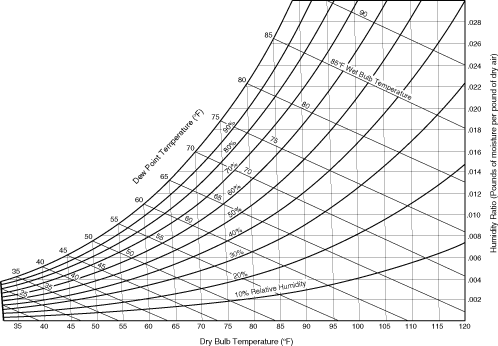
Figure 9.1.1. Psychometric chart
Credit: http://hem.dis.anl.gov/eehem/95/951108.html
As we know, varying amounts of moisture (in the gaseous or vapor form) exist in the air. Absolute Humidity is the actual amount of moisture that is contained in air. It is represented in the formula
\[ Absolute \, Humidity = \dfrac{Amount \, of \, Water \, Vapor \, (lb)}{Amount \, of \, Dry \, Air \, (lb)} \]
Relative humidity, in contrast, is the ratio of the amount of moisture in the air to the maximum amount of moisture the air can hold at a given temperature. It is represented in the formula
\[ Relative \, Humidity \, (at \, given \, temp) = \dfrac{Amount \, of \, Water \, Vapor \, (lb)}{Max \, Amount \, of \, Water \, Vapor \, Air \, can \, Hold} * 100 \]
Example 1
Calculate the relative humidity of air when the air contains 0.002 lb of moisture per pound of dry air while the maximum moisture air can hold at that temperature is 0.005 lb per lb of dry air.
- Answer
-
\[ Relative \, Humidity = \cfrac{\cfrac{0.002 \, lb}{lb \, dry \, air}}{\cfrac{0.005 \, lb}{lb \, of \, dry \, air}} * 100 = 40\% \]
Air is said to be saturated when the amount of water vapor in the air is the maximum possible at an existing temperature and pressure.
- Air is said to be saturated at 100% relative humidity when it contains the maximum amount of moisture possible at that specific temperature.
- Air holding half the maximum amount of moisture at a given temperature has a relative humidity of 50 percent.
When relative humidity reaches 100% or is saturated, moisture will condense, meaning the water vapor changes to liquid vapor.
Thus, the saturation level of air is related to the air's temperature. As air temperature increases (or becomes warmer), more water remains in a gas phase. As temperature decreases (or becomes colder), the water molecules slow down and it is more likely that they will condense onto nearby surfaces.
Dew Point is the temperature at which air reaches 100 percent relative humidity. If the air is cooled below dew point, moisture in the air condenses.
Moisture will condense on a surface whose temperature is below the dew point temperature of the air next to it. For air at a given absolute humidity, the colder the surface, the higher the relative humidity next to that surface. So the coldest surface in a room is the place where condensation will probably occur first (called the first condensing surface).
Important Point
Saturation is the maximum amount of water vapor in the air at an existing temperature and pressure. Air is said to be saturated at 100 percent relative humidity when it contains the maximum amount of moisture possible at that specific temperature.
Dew point is the temperature when air reaches 100% relative humidity.
The following video further explains humidity and condensation.


