1.4: Ions
- Page ID
- 31536
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
The idea of a covalent bond suggests equal sharing of the electron pair by the bonded atoms, but the brief discussion of polarity in Section 1-4 indicated that the sharing is not always equal. The relative electronegativity or electron-attracting power of atoms is of great importance in explaining chemical behavior, and is treated in detail in Chapters 9 and 10. Sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. Hence in common table salt (sodium chloride, NaCl), each sodium atom, Na, loses one electron (e-) to form a sodium ion, Na+. Each chlorine atom picks up one electron to become a chloride ion, Cl-:
-
- Na → Na+ + e-wordandword
 Cl2 + e- → Cl-
Cl2 + e- → Cl-
- Na → Na+ + e-wordandword
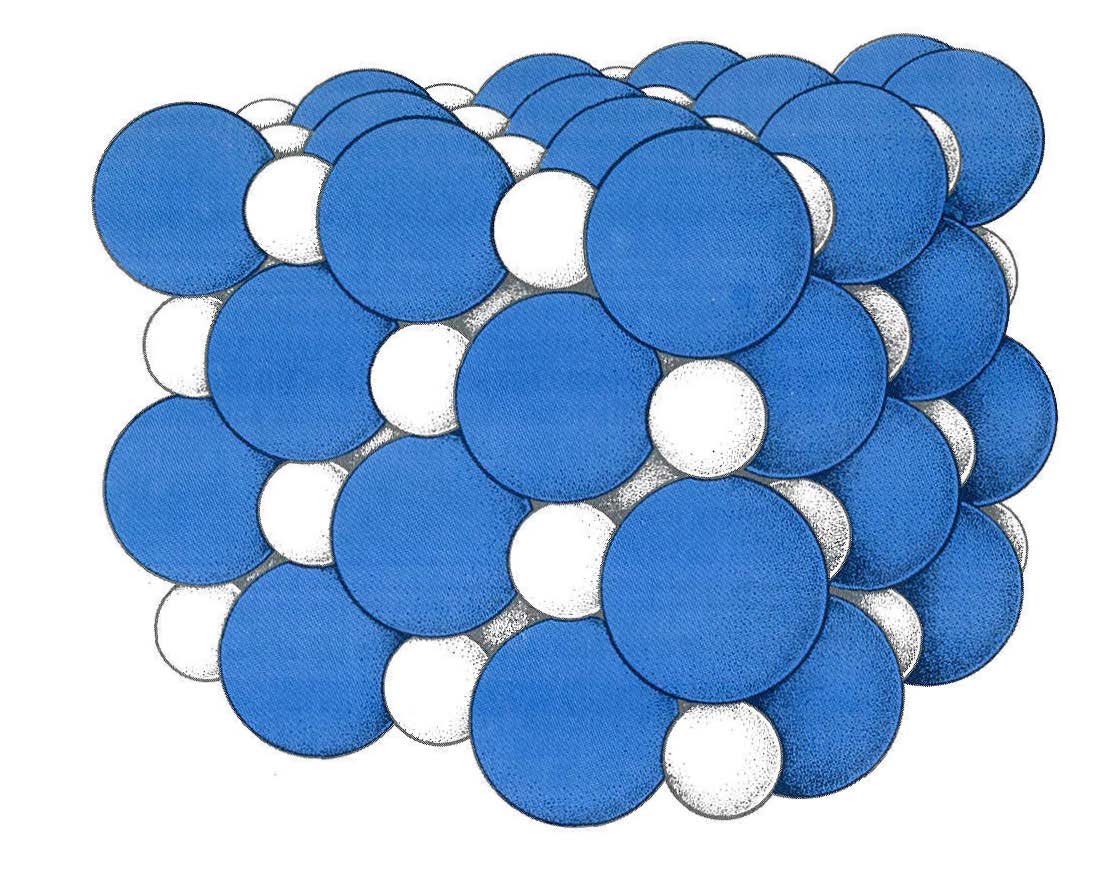
We write  Cl2 because free chlorine gas exists as diatomic (two-atom) molecules, not as free chlorine atoms. Solid sodium chloride (Figure 1-5) has sodium and chloride ions packed into a three-dimensional lattice in such a way that each positive Na+ ion is surrounded on four sides and top and bottom by negative Cl- ions, and each Cl- is similarly surrounded by six nearest neighbor Na+ ions. This is a particularly stable arrangement of positive and negative charges.
Cl2 because free chlorine gas exists as diatomic (two-atom) molecules, not as free chlorine atoms. Solid sodium chloride (Figure 1-5) has sodium and chloride ions packed into a three-dimensional lattice in such a way that each positive Na+ ion is surrounded on four sides and top and bottom by negative Cl- ions, and each Cl- is similarly surrounded by six nearest neighbor Na+ ions. This is a particularly stable arrangement of positive and negative charges.
Metals in general lose one to three electrons easily to become positively charged ions, or cations:
| Li | → | Li+ | + | e- | lithium ion | |
| Na | → | Na+ | + | e- | sodium ion | |
| K | → | K+ | + | e- | potassium ion | |
| Mg | → | Mg+2 | + | 2e- | magnesium ion | |
| Ca | → | Ca+2 | + | 2e- | calcium ion | |
| Al | → | Al+3 | + | 3e- | aluminum ion |
Some nonmetals, in contrast, pick up electrons to become negatively charged ions, or anions:
 F2 F2 |
+ | e- | → | F- | fluoride ion | |
 Cl2 Cl2 |
+ | e- | → | Cl- | chloride ion | |
 O2 O2 |
+ | 2e- | → | O-2 | oxide ion | |
 S2 S2 |
+ | 2e- | → | S-2 | sulfide ion |
Table 1-4 Some Simple Ions of Elements. From Dickerson, Gray, and Haight, Chemical Principles, 3rd ed., 1979. Retrieved from CaltechAUTHORS, Aug 10, 2020.
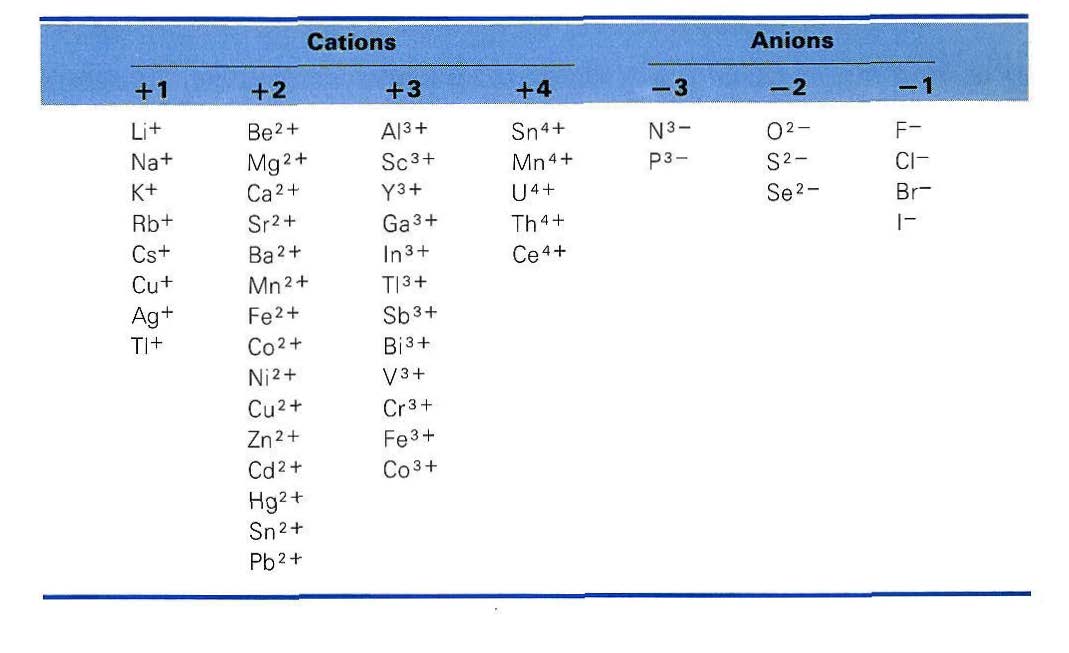
Other simple ions made from single atoms are shown in Table 1-4.
-
-
Fe2+ tt iron(II) or ferrous break Fe3+ tt iron(III) or ferric Cu+ tt copper(I) or cuprous break Cu2+ tt copper(II) or cupric Sn2+ tt tin(II) or stannous break Sn4+ tt tin(IV) or stannic
-
A salt is a compound made up of positive and negative ions. Because a salt must be electrically neutral, the total charge on its positive and negative ions must be zero. Since each ion of Sn2+ has a charge of +2, twice as many chloride ions with -1 charge each are required to produce a zero net charge. Hence the salt of Sn2+ and Cl- ions has the overall composition SnCl2, rather than SnCl or SnCl3. It is called stannous chloride or tin (II) chloride. The formula for stannic chloride or tin(IV) chloride is SnCl4.
Melting Points and Boiling Points of Salts
A salt crystal represents a particularly stable balance of positive and negative charges, with each type of ion being kept out of the way of others of like charge. Melting a salt crystal means upsetting this delicate balance of charges, and allowing mutually repelling ions to come closer together from time to time as the ions flow past one another. This disruption of structure requires large amounts of energy to accomplish, so the melting points of salts are higher than those of molecular solids. The melting points of two salts, sodium chloride (NaCI) and potassium sulfate (K2SO4), are compared in Table 1-7 with those of the elements from which the salts are made.
| Substance |
Chemical Formula |
Tm(°C) | Tb(°C) |
|---|---|---|---|
| Sodium metal | Na | 97.8 | 882.9 |
| Chlorine Gas | Cl2 | -101.0 | -34.6 |
| Sodium Chloride (salt) | NaCl | 801 | 1413 |
| Potassium metal | K | 64 | 774 |
| Sulfur | S | 119 | 445 |
| Oxygen gas | O2 | -218 | -183 |
| Potassium sulfate (salt) | K2SO4 | 1069 | 1689 |
Metallic sodium melts at 97.8°C, and solid chlorine melts at -101°C, but their combination, sodium chloride (common table salt), requires a temperature of 801°C before it will melt. Boiling or vaporizing a salt is even more difficult. The ions remain ions in the liquid state, tumbling past one another as in any other liquid; but before the gas phase can be attained, Na+ and CI- ions must pair off into neutral NaCl molecules. To accomplish this pairing, electrons have to be pulled away from CI- ions, which have a strong attraction for them, and pushed toward Na+ ions, which do not want them. The NaCl bond in sodium chloride vapor is extremely polar, with the electron pair skewed strongly toward the chlorine atom, but the separation still is not as complete as in Na+ and CI- ions. Much energy is required to push electrons where they are not wanted and to make NaCl molecules from Na+ and CI- ions, so high temperatures are required before this can happen. Hence the very high boiling points of salts in comparison with molecular compounds, as illustrated in Table 1-7.

