Page 13.2: Ozone Depletion
- Page ID
- 45385
The ozone depletion process begins when CFCs (chlorofluorocarbons) and other ozone-depleting substances (ODS) are emitted into the atmosphere. CFC molecules are extremely stable, and they do not dissolve in rain. After a period of several years, ODS molecules reach the stratosphere, about 10 kilometers above the Earth’s surface. CFCs were used by industry as refrigerants, degreasing solvents, and propellants.
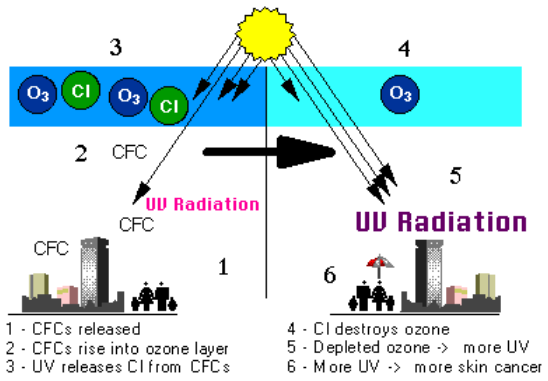 Figure \(\PageIndex{1}\). Strong UV light breaks apart the ODS molecule. CFCs, HCFCs, carbon tetrachloride, methyl chloroform, and other gases release chlorine atoms, and halons and methyl bromide release bromine atoms. It is these atoms that actually destroy ozone, not the intact ODS molecule. It is estimated that one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Credit: NASA GSFC.
Figure \(\PageIndex{1}\). Strong UV light breaks apart the ODS molecule. CFCs, HCFCs, carbon tetrachloride, methyl chloroform, and other gases release chlorine atoms, and halons and methyl bromide release bromine atoms. It is these atoms that actually destroy ozone, not the intact ODS molecule. It is estimated that one chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Credit: NASA GSFC.
Ozone (O3) is constantly produced and destroyed in a natural cycle, as shown in Figure \(\PageIndex{1}\). However, the overall amount of ozone is essentially stable. This balance can be thought of as a stream’s depth at a particular location. Although individual water molecules are moving past the observer, the total depth remains constant. Similarly, while ozone production and destruction are balanced, ozone levels remain stable. This was the situation until the past several decades. Large increases in stratospheric ODS, however, have upset that balance. In effect, they are removing ozone faster than natural ozone creation reactions can keep up. Therefore, ozone levels fall.
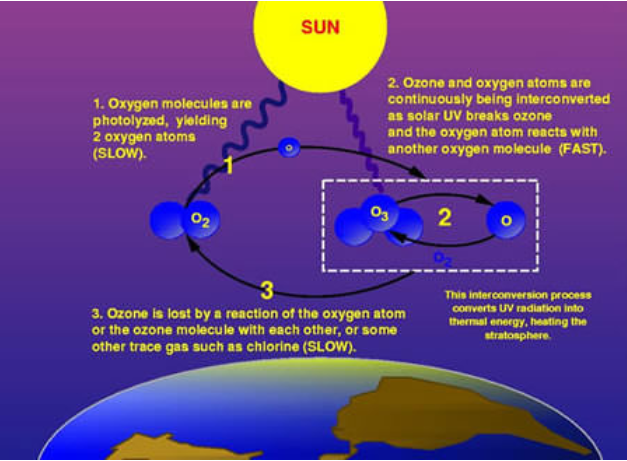 Figure \(\PageIndex{2}\). Because ozone filters out harmful UVB radiation, less ozone means higher UVB levels at the surface. The more the depletion, the larger the increase in incoming UVB. UVB has been linked to skin cancer, cataracts, damage to materials like plastics, and harm to certain crops and marine organisms. Although some UVB reaches the surface even without ozone depletion, its harmful effects will increase as a result of this problem.
Figure \(\PageIndex{2}\). Because ozone filters out harmful UVB radiation, less ozone means higher UVB levels at the surface. The more the depletion, the larger the increase in incoming UVB. UVB has been linked to skin cancer, cataracts, damage to materials like plastics, and harm to certain crops and marine organisms. Although some UVB reaches the surface even without ozone depletion, its harmful effects will increase as a result of this problem.
Policies to Reduce Ozone Destruction
One success story in reducing pollutants that harm the atmosphere concerns ozone-destroying chemicals. In 1973, scientists calculated that CFCs could reach the stratosphere and break apart. This would release chlorine atoms, which would then destroy ozone. Based only on their calculations, the United States and most Scandinavian countries banned CFCs in spray cans in 1978. More confirmation that CFCs break down ozone was needed before more was done to reduce production of ozone-destroying chemicals. In 1985, members of the British Antarctic Survey reported that a 50% reduction in the ozone layer had been found over Antarctica in the previous three springs.
Two years after the British Antarctic Survey report, the “Montreal Protocol on Substances that Deplete the Ozone Layer” was ratified by nations all over the world. The Montreal Protocolcontrols the production and consumption of 96 chemicals that damage the ozone layer (Figure \(\PageIndex{3}\)). CFCs have been mostly phased out since 1995, although they were used in developing nations until 2010. Some of the less hazardous substances will not be phased out until 2030. The Protocol also requires that wealthier nations donate money to develop technologies that will replace these chemicals.
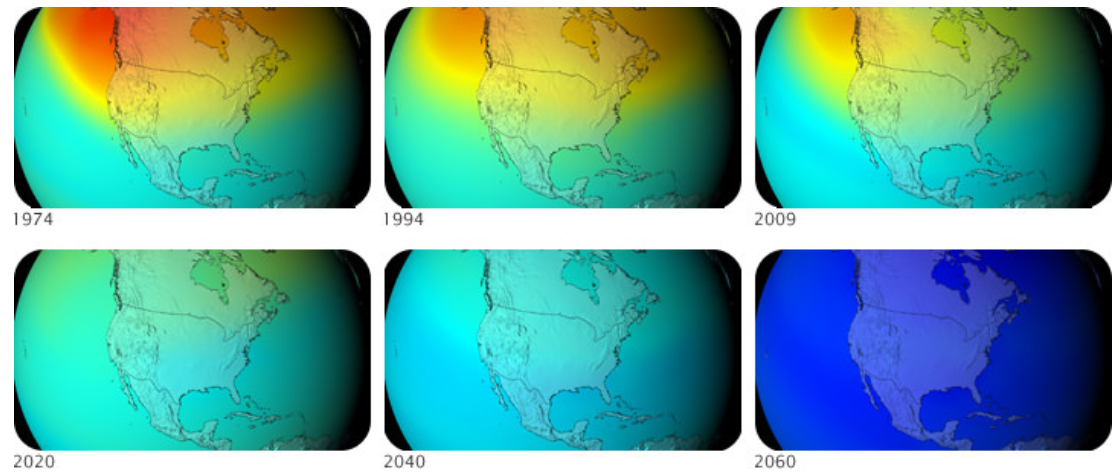 Figure \(\PageIndex{3}\). Ozone levels over North America decreased between 1974 and 2009. Models of the future predict what ozone levels would have been if CFCs were not being phased out. Warmer colors indicate more ozone.
Figure \(\PageIndex{3}\). Ozone levels over North America decreased between 1974 and 2009. Models of the future predict what ozone levels would have been if CFCs were not being phased out. Warmer colors indicate more ozone.
Because CFCs take many years to reach the stratosphere and can survive there a long time before they break down, the ozone hole will probably continue to grow for some time before it begins to shrink. The ozone layer will reach the same levels it had before 1980 around 2068 and 1950 levels in one or two centuries.
Health and Environmental Effects of Ozone Layer Depletion
There are three types of UV light: UVA, UVB, and UVC. Reductions in stratospheric ozone levels will lead to higher levels of UVB reaching the Earth’s surface. The sun’s output of UVB does not change; rather, less ozone means less protection, and hence more UVB reaches the Earth. Studies have shown that in the Antarctic, the amount of UVB measured at the surface can double during the annual ozone hole.
Laboratory and epidemiological studies demonstrate that UVB causes non-melanoma skin cancers and plays a major role in malignant melanoma development. In addition, UVB has been linked to cataracts, a clouding of the eye’s lens. All sunlight contains some UVB, even with normal stratospheric ozone levels. Therefore, it is always important to protect your skin and eyes from the sun. Ozone layer depletion increases the amount of UVB and the risk of health effects.
UVB is generally harmful to cells, and therefore all organisms. UVB cannot penetrate into an organism very far and thus tends to only impact skin cells. Microbes like bacteria, however, are composed of only one cell and can therefore be harmed by UVB,
Contributors and Attributions
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher.


