5.2: Biomass Carbohydrate Tutorial
- Page ID
- 48568
5.2 Biomass Carbohydrate Tutorial
When the word carbohydrate is used, I typically think of the carbohydrates in food. Carbohydrates are the sugars and complex units composed of sugars. This section will describe each.
Sugars are also called saccharides. Monomer units are single units of sugars called monosaccharides. Dimer units are double units of sugars called disaccharides. Polymers contain multiple units of monomers and dimers and are called polysaccharides.
So, what are typical monosaccharides? They are made up of a molecule that is in a ring structure with carbons and oxygen. Figure 5.9a shows the structure of glucose; it is made up of C6H12O6. Glucose is distinguished by its structure: five carbons in the ring with one oxygen; CH2OH attached to a carbon; and OH and H groups attached to the other carbons. This sugar is known as blood sugar and is an immediate source of energy for cellular respiration. Figure 5.9b shows galactose next to glucose, and we can see that galactose is almost like glucose, except on the No. 4 carbon the OH and H are an isomer and just slightly different (highlighted in red on the galactose molecule). Galactose is a sugar monomer in milk and yogurt. Figure 5.9c shows fructose; while it still has a similar chemical formula as glucose (C6H12O5), it is a five-membered ring with carbons and oxygens, but two CH2OH groups. This is a sugar found in honey and fruits.
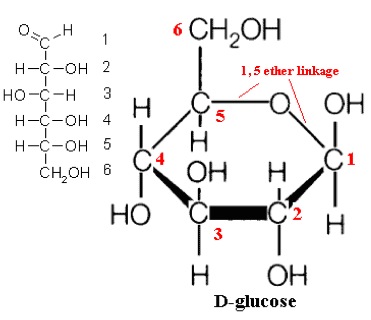
Credit: Palaeos.com
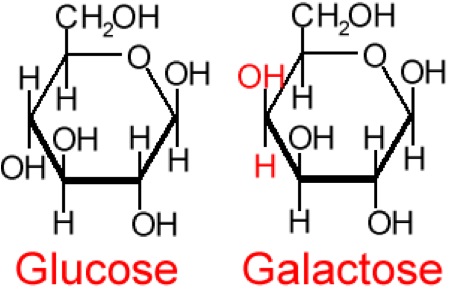
Credit: Palaeos.com
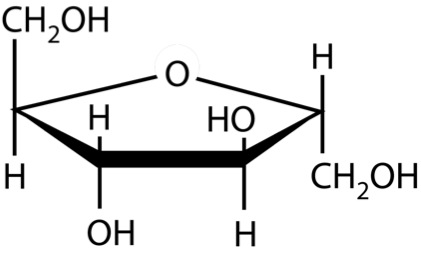
Credit: Palaeos.com
We also have disaccharides as sugars in food. Disaccharides are dimers of the monomers we just discussed and are shown below. One of the most common disaccharides is sucrose, which is common table sugar and is shown in Figure 5.10a. It is a dimer of glucose and fructose. Another common sugar dimer is lactose. It is the major sugar in milk and a dimer of galactose and glucose (see Figure 5.10b). Maltose (5.10c) is also a sugar dimer but is a product of starch digestion. It is a dimer made up of glucose and glucose. In the next section, we will discuss what starch and cellulose are composed of in order to see why maltose is a product of starch digestion.
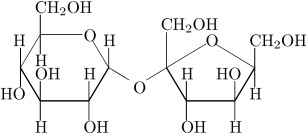
Credit: World of Molecules
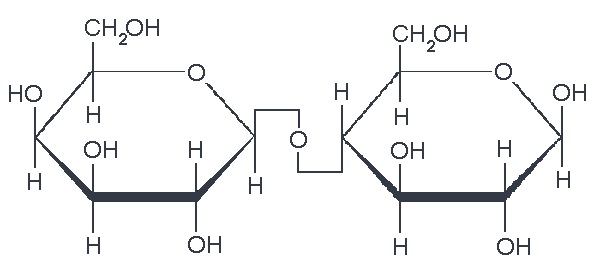
Credit: Optushome.com
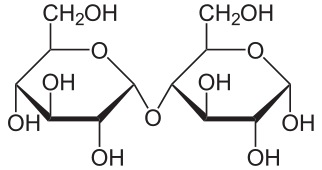
Carbohydrate structure
All carbohydrate polymers are monomers that connect with what is called a glycosidic bond. For example, sucrose is a dimer of glucose and fructose. In order for the bond to form, there is a loss of H and OH. So, another way to show this is:
C12H22O11 = 2 C6H12O6 − H2O
And as dimers can form, polymers will form and are called polysaccharides. Typical polysaccharides include 1) glycogen, 2) starch, and 3) cellulose. Glycogen is a molecule in which animals store glucose by polymerizing glucose, as shown in Figure 5.11.
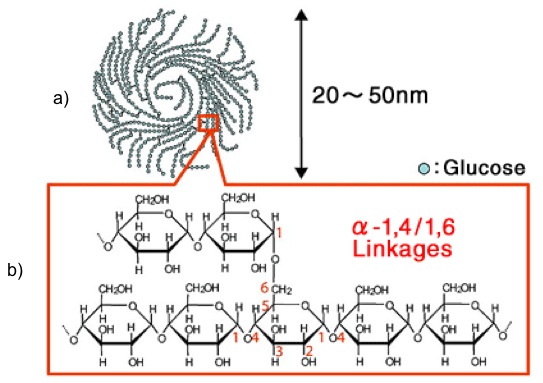
Credit: Glico
Starches are similar to glycogen, with a little bit different structure. Starch is composed of two polymeric molecules, amylose and amylopectin. The structures of both are shown in Figure 5.12a and 5.12b.
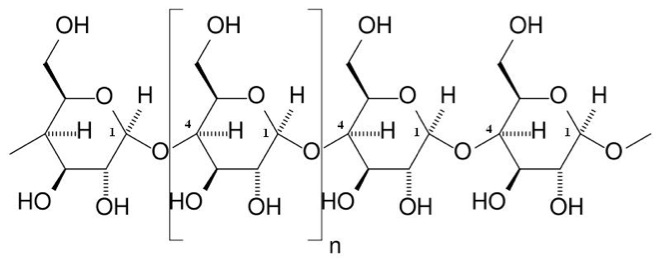
Credit: Marshall.edu
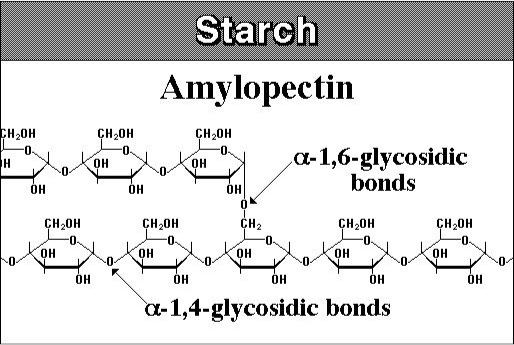
Credit: nutritionalbiochem.blogspot.com
About 20% of starch is made up of amylose and is a straight-chain that forms into a helical shape with α-1,4 glycosidic bonds and the rest of the starch is amylopectin, which is branched with α-1,4, and α-1,6 glycosidic bonds. Figure 5.13 shows the structure of cellulose. Cellulose is a major molecule in the plant world; it is also the single most abundant molecule in the biosphere. It is a polymer of glucose and has connectors of the glucose molecule that are different from starch; the linkages are β-1,4 glycosidic bonds. The polymer of cellulose is such that it can form tight hydrogen bonds with oxygen, so it is more rigid and crystalline than starch molecules. The rigidity makes it difficult to break down.
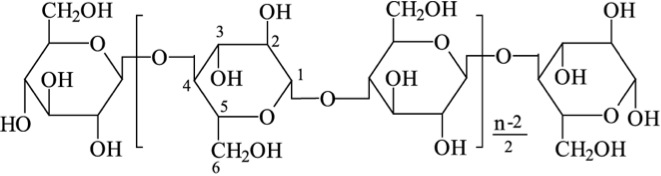
Credit: Z.H. Gao, J.Y. Gu, X‐M. Wang, Z.G. Li, X.D. Bai, (2005) "FTIR and XPS study of the reaction of phenyl isocyanate and cellulose with different moisture contents," Pigment & Resin Technology, Vol. 34 Iss: 5, pp.282 - 289


