3: Critical Properties and Acentric Factor
- Page ID
- 8068
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Distillation Science (a blend of Chemistry and Chemical Engineering)
This is Part III, Critical Properties and Acentric Factor of a ten-part series of technical articles on Distillation Science, as is currently practiced on an industrial level. See also Part I, Overview for introductory comments, the scope of the article series, and nomenclature. The goal of this article is to explain how the critical properties and acentric factor are used, and how they may be estimated when reliable values are not available.
As discussed in Part II, the more basic vapor pressure equations have limitations that prevent them from being used in many modern industrial distillation systems, specifically at higher pressures. The concepts in this article will be used in Part IV, to introduce an improved vapor pressure equation, which removes the limitations of basic vapor pressure equations.
To implement the more sophisticated VP relationships and inter-compare the constants in Part IV, experimentally measured P vs T data needs to be put into reduced form. This is done only with the knowledge of the critical point, which is that singularity on the saturation line where the liquid and vapor phases become one. The critical temperature and pressure are identified as Tc and Pc. At the critical point, the specific molar volume is Vc.
\[T_{r} = T/T_{c} \label{3-1} \]
\(P_{r} = P/P_{c} \nonumber\)
\(V_{r} = V/V_{c} \nonumber\)
(note that reduced properties have the advantage of being dimensionless)
Using reduced variables Tr, Pr and Vr from Equation (\ref{3-1}) also allows introduction to the concept of the "Law of Corresponding States" ( which is really not a scientific law per se, but more of a generally followed relationship). This "law" expresses the generalization that those properties dependent on intermolecular forces are related to the critical properties in the same way for all fluids. This concept underlies the development of several later articles of this series on Distillation Science, including Parts IV through VII.
The critical compressibility is Zc, as defined as:
\[Z_{c}= \frac {P_{c}V_{c}}{RT_{c}} \label{3-2} \] which is also dimensionless.
Two other properties are important to note:
- \(T_b\) is defined as the atmospheric pressure boiling point of a fluid, with \(T_{br}\) as the reduced value of the atmospheric boiling point
- \(ω\) is the acentric factor, which is a measure of molecular complexity as defined as:
\[\omega=-\log_{10}(VP)-1 \label{3-3} \] where the VP (in atmospheres) is evaluated at Tr= 0.7
The term “acentric factor” comes from Kenneth Pfizer's observation in 1955 that compact and near-spherical molecules have close to zero values when their ω is calculated. For example, neon, argon and krypton have ω values of -0.04, 0.00 and 0.00, respectively (helium has a more slightly negative ω, the value depending on isotope). Methane (CH4) has a ω of 0.011; and the molecularly larger silane (SiH4) has an ω of 0.099. An even larger and more complex molecule, like carbon tetrachloride (CCl4) has an ω of 0.193; whereas silicon tetrachloride (SiCl4) has an ω of 0.248. So valuation of the acentric factor from VP data and knowledge of the critical point is a good validation check against the known molecular size and shape.
For Equations of State (see Part V) that are more advanced than Van der Waals, the acentric factor is a required property, along with critical temperature and pressure. It is also used along with critical properties in the estimation of binary interaction parameters in Part VII.
As mentioned in Part II, the basic VP relationships have acceptable accuracy to obtain good values of Tb from available data. Experimental data is often taken near atmospheric pressure, but not exactly at one atmosphere absolute (i.e, 760 mmHg = 760 Torr = 101.325 Pa). Instead of blindly accepting a value of Tb from a handbook or single website, the practicing scientist or engineer should consider the data’s source and reliability. There is ready internet availability of atmospheric boiling points, critical property data, and acentric factors, especially on the NIST WebBook and global websites like Dechema, Infotherm (recently acquired by John Wiley and Sons from FIZ Chemie Berlin).
A recommended way to sort through the dizzying amount of critical property data (i.e., Tc , Pc , and Vc) is to use the correlation techniques of Lyderson ("Estimation of Critical Properties of Organic Compounds", University of Wisconsin College of Engineering, 1955) and others, which are specifically geared toward organic compounds. With some algebraic manipulation and adjustment of Lyderson’s parameters (since the usage intent here is for polar compounds that are not organic, but are not ionic either), and based on data across homologues, the following relationships seem to hold and are recommended for both validating questionable data or filling in “holes” of no data:
For Tb, the general correlating relationship within a homologue ( e.g., from silane to silicon tetrachloride, or from phosphine to trichlorophosphine) :
\[(T_{b}\times MW)^n= A+B\times MW \label{3-4} \]
where MW is the molecular weight. For chlorosilanes, chloromethanes, chlorophosphines, etc, this relationship shows a best fit with an exponent “n” of 0.8320. The constants A and B for the homologue are typically set by the hydride and chloride, since that data is usually more reliable. Where the core atom is not just carbon or silicon, but a blend (i.e, a methyl silane), an exponent of 0.8455 fits the data a bit better. This contrasts with organic compounds, whose data tends to fit best with somewhat lower exponents that are closer to 0.80.
For Tc (critical temperature), the best correlation within a homologue is found to be:
\[\frac {T_{c}-T_{b}}{T_{c}}= A+B\times MW \label{3-5} \]
where MW is the molecular weight and the constants A and B for the homologue are typically set by the hydride and chloride, as long as there is no organic content and the fluids are monomeric.
For Pc (critical temperature), use
\[(\frac {MW}{P_{c}})^n= A+B\times MW \label{3-6} \]
where MW is the molecular weight, exponent "n" = 0.5672, and constants A and B for the homologue are typically set by the hydride and chloride (as long as there is no organic content and the fluids are monomeric). For a pure organic compound, the exponent "n" should be Lyderson’s suggested 0.5000; for methyl silanes (e.g, dimethyl silane) the best exponent is 0.4550; and for Group III (dimeric bridge-bonded) compounds the best exponent fit is 1.03-1.08 ( 1.03 for di-gallanes and 1.08 for diboranes).
For Vc (critical molar volume), Lyderson’s rule of
\[V_{c}=A+B\times MW \label{3-7} \]
is probably still the best, where MW is the molecular weight and constants A and B for the homologue are typically set by the hydride and chloride. For some homologues, there is a possibility that the Vc vs MW relationship is not exactly linear, but rather has a slight concave quadratic nature; but the data is rarely good enough to determine such. Of all the critical properties, Vc is the hardest to experimentally measure, and is frequently estimated.
There is a way to get around some of the uncertainty with Vc, and that is to validate (or make minor Vc adjustments) based on the pattern of Zc values in the homologue where
\[Z_{c}= \frac{P_{c}\times V_{c}}{R\times T_{c}} \label{3-8} \]
Within each homologue there is a characteristic “check-mark” pattern to Zc values, when plotted against the number of hydrogen to chlorine swaps of the homologue (i.e., the homologue’s hydride has zero swaps, the monochloride one swap, the dichloride two swaps, etc). See below example data plot Figure 3-1 for the chlorosilane and chlorophosphine homologues.
The homologue’s hydride (which tends to be a fairly spherical shaped molecule with minimal dipole moment) typically has a Zc in the range of 0.27-0.29. The first swap replaces a small “H” atom with a larger “Cl” atom, and re-orients the molecular shape to have a significant dipole, dropping the Zc value by about 10%. Then the next swaps reduce the dipole, until the molecular shape returns closer to spherical, although significantly larger in diameter. Typically the Zc of the completely chlorinated homologue fluid has a slightly larger Zc than the homologue’s hydride.
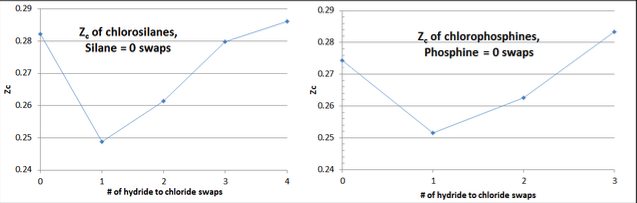
To the extent that this “checkmark” shape is not observed in plotting calculated Zc values per Equation 3-8, the “culprit” is almost always the value of Vc, which allows for some correction. The value of Zc is known to have a significant relationship to molecular shape and complexity, although the stronger relationship between Zc and the acentric factor, ω, is not so easily generalized as in organic compounds.
The importance of accurate values of Tb , Tc , Pc (and ω to an extent) is in calculating vapor pressure using the advanced relationship of Part IV. Having an accurate value of ω is more important, along with Tc, Pc in calculating the Equation of State for a fluid, in Part V. Having good values for ω and Vc are important to the estimation of binary interaction parameters in Part VII.
When two different types of swaps are made in a slightly polar molecule, an alternate technique is used to best correlate fluid properties (Tb , Tc , Pc , Vc , and ω), with a good example being methyl chlorosilanes. With methyl chlorosilanes, one or more methyl groups are substituted for hydrogen atoms (i.e., Si-H swapped to Si-CH3), with the remaining Si-H bonding possibly swapped to Si-Cl. Another example would be swapping C-H for C-Cl and C-F bonding in chloro-fluoro methanes. There is no concise formula that governs such a dual substitution. Instead the concept of neural networks is used.
In this technique, the two base curves are laid out from the same starting compound (silane in the example below), and on each base curve the pertinent physical property values shown for each substituted homologue (chlorosilanes and methyl silanes being the base curves in the example below) vs molecular weight. For each amount of combined substitution (100%, 75% and 50% substitution = 0%, 25%, and 50% Si-H remaining) the data points are connected to their respective base curves. For example, on the 75% substitution amount, one would curve-connect trichlorosilane, methyl dichlorosilane, dimethyl monochlorosilane, and trimethyl silane.
Additional curve-connections could be made for values of constant amount of chlorine or methyl substitution (e.g., curve-connecting monochlorosilane, methyl monochlorosilane, dimethyl monochlorosilane, and trimethyl monochlorosilane). Using this method, the data on the dual-substituted fluids can be validated and adjustments made so that the curve-connections are smooth.
In the example in below Figure 3-2, the values of Pc for methyl monochlorosilane (sic, me-mono) and dimethyl monochlorosilane (sic, di-me mono) are hardened up from rough measurements.
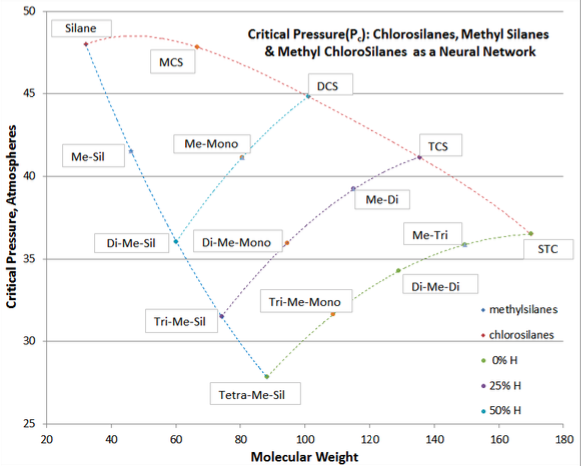
The value of the neural network technique is to make adjustments where data is poor or the estimation is of questionable accuracy. The presumption of neural networks is that for a class of compound, the continuum of structural changes should be uniform and internally consistent (although not necessarily linear).
Neural networks were constructed for all the pertinent properties of methyl chlorosilanes, and the technique found to be rather simple to deal with graphically using MS Excel. The resulting values were included in Table 3-3 below, for chlorosilanes and their common impurities, whose properties were validated using the correlating techniques of Equation (\ref{3-4}) through Equation (\ref{3-8}).
|
Fluid |
MW |
Tb |
Tc |
Pc |
Vc |
Zc |
ω |
|---|---|---|---|---|---|---|---|
|
Group III(A) |
|||||||
|
B2H6 |
27.670 |
180.54 |
289.70 |
39.58 |
173.10 |
0.2882 |
0.1254 |
|
B2H5Cl |
62.119ǂ |
216.37 |
346.33 |
37.13 |
189.92 |
0.2481 |
0.1283 |
|
BH2Cl as monomer |
42.284 |
237.72 |
379.56 |
37.10 |
206.73 |
0.2463 |
0.1315 |
|
BHCl2 as monomer |
82.722 |
266.07 |
422.71 |
37.63 |
240.37 |
0.2608 |
0.1389 |
|
B2HCl5 hypothetic |
199.893ǂ |
276.67 |
438.47 |
37.92 |
257.18 |
0.2711 |
0.1433 |
|
BCl3 |
117.169 |
285.88 |
451.95 |
38.20 |
274.00 |
0.2822 |
0.1468 |
|
AlCl3 as monomer |
133.341 |
466.86* |
625.70 |
26.00 |
261.80 |
0.1326 |
0.3474 |
|
Ga2H4Cl2 as dimer |
214.383ǂ |
350.86 |
545.93 |
40.94 |
231.9 |
0.2120 |
0.2726 |
|
Ga2H2Cl4 as dimer |
283.273ǂ |
421.79 |
639.69 |
41.02 |
247.5 |
0.1943 |
0.3525 |
|
Ga2Cl6 as dimer |
352.162ǂ |
473.49 |
694.00 |
37.70 |
263.0 |
0.1741 |
0.4504 |
|
Group IV(A) |
|||||||
|
SiH4 |
32.117 |
161.75 |
269.65 |
47.99 |
130.07 |
0.2821 |
0.09860 |
|
SiH3Cl |
66.562 |
242.75 |
396.65 |
47.82 |
169.32 |
0.2488 |
0.1252 |
|
SiH2Cl2 |
101.007 |
281.45 |
449.45 |
44.83 |
215.05 |
0.2614 |
0.1589 |
|
SiHCl3 |
135.452 |
306.15 |
479.15 |
41.15 |
267.28 |
0.2797 |
0.2090 |
|
SiCl4 |
169.896 |
330.72 |
506.95 |
36.50 |
326.00 |
0.2860 |
0.2482 |
|
SiH3(CH3) |
46.144 |
216.48 |
348.35 |
41.53 |
185.20 |
0.2691 |
0.1264 |
|
SiH2Cl(CH3) |
80.589 |
278.82 |
439.11 |
41.16 |
233.90 |
0.2672 |
0.1793 |
|
SiHCl2(CH3) |
115.034 |
314.17 |
489.18 |
39.25 |
287.81 |
0.2814 |
0.2269 |
|
SiCl3(CH3) |
149.479 |
339.72 |
517.61 |
35.86 |
345.70 |
0.2919 |
0.2655 |
|
SiH2(CH3)2 |
60.169 |
252.86 |
399.17 |
36.05 |
245.84 |
0.2706 |
0.1604 |
|
SiHCl(CH3)2 |
94.615 |
305.30 |
471.35 |
35.94 |
300.32 |
0.2791 |
0.2264 |
|
SiCl2(CH3)2 |
129.061 |
342.89 |
519.21 |
34.26 |
358.20 |
0.2880 |
0.2740 |
|
GeH4 |
76.642 |
184.93 |
307.98 |
54.77 |
128.28 |
0.2780 |
0.1270 |
|
GeH3Cl |
111.087 |
302.91 |
495.10 |
49.95 |
180.17 |
0.2215 |
0.1476 |
|
GeH2Cl2 |
145.532 |
334.60 |
536.93 |
45.36 |
236.71 |
0.2437 |
0.1721 |
|
GeHCl3 |
179.976 |
343.54 |
541.41 |
41.42 |
283.96 |
0.2647 |
0.2012 |
|
GeCl4 |
214.421 |
357.28 |
553.16 |
38.10 |
335.86 |
0.2819 |
0.2334 |
|
SnH4 |
122.742 |
221.07 |
360.20 |
51.70 |
152.90 |
0.2674 |
0.1619 |
|
SnCl4 |
260.521 |
387.21 |
591.85 |
36.95 |
351.20 |
0.2672 |
0.2625 |
|
Group V(A) |
|||||||
|
PH3 |
33.998 |
185.41 |
324.75 |
64.51 |
113.33 |
0.2743 |
0.03052 |
|
PH2Cl |
68.443 |
273.09 |
463.73 |
62.29 |
153.63 |
0.2515 |
0.0750 |
|
PHCl2 |
102.888 |
318.44 |
524.74 |
55.82 |
202.52 |
0.2625 |
0.1362 |
|
PCl3 |
137.333 |
349.25 |
558.95 |
50.00 |
260.00 |
0.2834 |
0.2117 |
|
POCl3 |
153.331 |
379.00 |
605.21 |
47.59 |
276.00 |
0.2645 |
0.1993 |
Table 3-3, Continued
|
AsH3 |
77.945 |
210.73 |
373.00 |
65.12 |
132.50 |
0.2819 |
0.01341 |
|
AsH2Cl |
112.390 |
300.41 |
515.99 |
64.33 |
174.85 |
0.2657 |
0.0573 |
|
AsHCl2 |
146.835 |
359.66 |
600.00 |
61.57 |
218.29 |
0.2730 |
0.1153 |
|
AsCl3 |
181.281 |
403.30 |
654.00 |
58.35 |
259.56 |
0.2822 |
0.1875 |
|
SbH3 |
124.781 |
256.09 |
446.20 |
66.61 |
157.20 |
0.2860 |
0.01659 |
|
SbCl3 |
228.115 |
794.05 |
794.05 |
68.85 |
268.00 |
0.2832 |
0.3309 |
|
n-pentane |
72.149 |
309.16 |
470.05 |
32.86 |
310.0 |
0.2641 |
0.2393 |
|
iso-pentane |
72.149 |
300.82 |
460.56 |
33.17 |
307.1 |
.2695 |
0.2147 |
In above Table 3-3, those entries that are marked with a ǂ have their molecular weight given as the dimer. For AlCl3, the asterisk on the Tb entry is for the best correlating value for the Part IV VP equation, even though it is below the triple point. Group IIIA compounds can feature “bridge-bonding” and can be either monomeric or dimeric. The two pentane entries at the end of the table are included because of their significance as electronic impurities, and to show that the techniques can be extended to organics.
With Group IIIA, some compounds are not included in a homologue simply because they are impossible structurally or are unstable. An example would be B2H3Cl3, which would put too much strain on the B-B bridge-bond. Another is AlH3 which cannot exist as a stand-alone liquid with vapor pressure at normal processing conditions, but tends to form stable hydrides with Group I metals such as LiAlH4. However, B2HCl5 is included (but noted as hypothetic), since some researchers claim to have seen its presence in low levels of TCS.


