2.1: Definitions of Reaction Rate and Extent of Reactions
- Page ID
- 101145
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By the end of this section, you should be able to:
- Define Reaction rate and r eaction extent
Calculate the reaction rate for the reaction, the rate of formation of compounds and the reaction extent
Reaction Rate
A reaction rate shows the rates of production of a chemical species. It can also show the rate of consumption of a species; for example, a reactant. In general though, we want an overall common reaction rate to describe changes in a chemical system.
Let’s look at an example. Say we have reaction represented as: \(A + 2B → 3C + D\)
For this system reaction rate can be expressed as follows:
\[r = \frac{d[D]}{dt}\]
The reaction rate is represented by the letter “r” or the Greek letter upsilon “\(\upsilon\)”
NOTE: I will stick with r as upsilon looks like “\(\nu\)” which we will use to represent the stoichiometric coefficient
Above we have written the reaction rate as if a substance with a coefficient of 1 was reacting (or being produced). This is the typical form of an overall reaction rate describing a reaction.
Rearranging the above equation, we can find the rate of production/consumption for any species based on this overall reaction rate, note that stoichiometric coefficients are positive for products and negative for reactants:
\[r_{A}=\frac{d[A]}{dt}=-\nu_{A}×r=(-1)×r\]
\[r_{B}=\frac{d[B]}{dt}=-\nu_{B}×r=(-2)×r\]
\[r_{C}=\frac{d[C]}{dt}=\nu_{C}×r=3×r\]
\[r_{D}=\frac{d[D]}{dt}=\nu_{D}×r=1×r\]
The general equation for calculating the reaction rate:
General notation: J is used to denote any compound involved in the reaction.
| \(r = \frac{1}{\nu}\frac{d[J]}{dt}\) |
Reaction rate at a given time can also be found from the graph of concentration of components in a system vs. time:
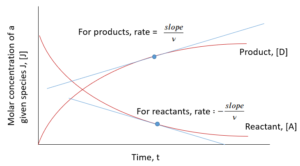
If we have the reaction
\[2 NOBr_{(g)} ⇌ 2 NO_{(g)} + Br_{2(g)}\]
and we measure that the rate of formation of NO is 1.6 mmol/(L·s), what are the overall reaction rate, and the rate of formation of \(Br_{2}\) and \(N\!O\!Br\)?
Solution
Add example text here.
Step 1: Determine the overall reaction rate from the rate of formation for NO.
\begin{align*}
r & = \frac{1}{\nu_{j}} \frac{d[NO]}{dt} \\
& = \frac{1}{2} \left( 1.6 \frac{mmol}{L·s}\right)\\
& = 0.8\frac{mmol}{L·s}
\end{align*}
Step 2: Use the reaction rate to determine rate of formation for the other compounds
NOTE: rate of formation is positive for products and negative for reactants
\begin{align*}
\frac{d[Br_{2}]}{dt}& = r \\
& = 0.8\frac{mmol}{L·s}
\end{align*}
\begin{align*}
\frac{d[NOBr]}{dt}& = -2r \\
& = -1.6\frac{mmol}{L·s}
\end{align*}
Reaction rates can be given in a variety of units over time. In this class we will just explore molarity and partial pressure, although other forms exist.
Molarity – molar concentration – expressed in units of \(\frac{mol}{volume * time}\) (eg. \(\frac{mol}{L*s}\) )
Partial pressure – the pressure produced by one gaseous component if it occupies the whole system volume at the same temperature, commonly used for gasses – units of \(\frac{pressure}{time}\)( eg. \(\frac{Pa}{s}\) )
Extent of Reaction
We use the extent of reaction (\(\xi\)) to describe the change in an amount of a reacting speicies J.
| \(d n_{j} = \nu_{j} d\xi\) |
where:
- \(dn_{j}\) = change in the number of moles of a certain substance
- \(\nu_{j}\) = the stoichiometric coefficient
- \(d\xi\) = the extent of reaction
We can get a relationship between the reaction extent and the rate of reaction when the system volume is constant:
| \(r = \frac{1}{V} \frac{d\xi}{dt} = \frac{1}{\nu_{j}} \frac{1}{V} \frac{dn_{j}}{dt}\) |
where:
\[V\]

