9.2: Measures of the Ability of Charges to Flow
- Page ID
- 18992
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The idea of flow of charges is fundamental to both electrical engineering and chemistry. However, electrical engineers and chemists make different assumptions, and they use different notations to describe closely related phenomena. Engineers prefer to work with solids because solids are durable. Electrical engineers assume all discussions involve solids unless otherwise specified. Chemists, however, are quite interested in, and assume all discussions involve, liquids, with special focus on aqueous solutions. Batteries and fuel cells typically involve charge flow through both liquids and solids, so to understand these devices, we have to be familiar with notations and assumptions from both fields of study.
In solid conductors, valence electrons flow. Inner shell electrons are assumed to be so tightly bound to atoms that their movements can be ignored. Nuclei are so much heavier than electrons that their movements can also be ignored. In solid semiconductors, both valence electrons and holes flow. Electrical engineers measure the ability of charges to flow in materials by the electrical conductivity.
Positive and negative ions can flow more easily in liquids than solids, so chemists are concerned with the flow of both electrons and ions. Semiconductor physicists tend to use the terms electrical conductivity, resistivity, Fermi level, and energy gap. Chemists are so interested in the ability of charges to flow that they have many interrelated measures to describe it. We'll discuss the following measures:
- Mulliken electronegativity
- Ionization energy
- Electron affinity
- Electronegativity
- Chemical potential
- Chemical hardness
- Redox potential
- pH
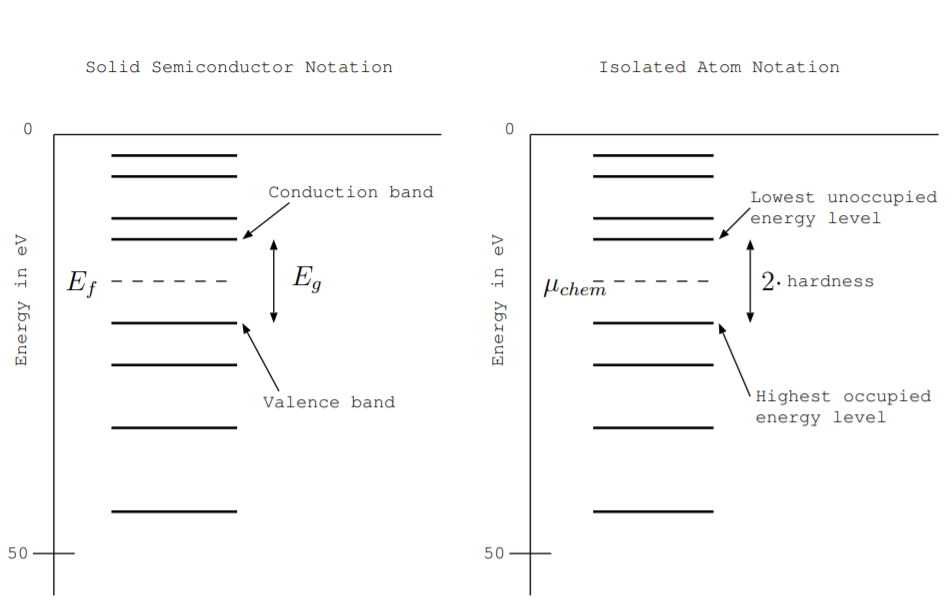
Electrical Conductivity, Fermi Energy Level, and Energy Gap Revisited
Electrical conductivity \(\sigma\) is measured in units \(\frac{1}{\Omega \cdot m}\), and it was discussed in Sec. 8.6.2. The inverse is resistivity \(\rho = \frac{1}{\sigma}\) measured in \(\Omega \cdot m\). Electrical conductivity and resistivity are measures of the ability of electrons to flow through a material. As described by Equation 8.5.10, electrical conductivity is directly proportional to the number of charges present and the mobility of the charges. Conductors have large electrical conductivity, and insulators have small electrical conductivity. These measures can describe liquids and gases as well as solids. Also, gases, liquids, and solids can all be classified as conductors, dielectrics, or semiconductors.
Fermi energy level, energy gap, valence band, and conduction band were defined in Section 6.3. The left part of Fig. \(\PageIndex{1}\) shows an energy level diagram zoomed in so that only some levels are shown, and these terms are illustrated in the figure. At \(T = 0\) K, energy levels are filled up to some level called the valence band. The energy level above it, which is unfilled or partially unfilled, is called the conduction band. The amount of energy needed to completely remove an electron from the valence band is represented by the vertical distance from that energy level to the ground state, labeled 0 eV, at the top of the figure. The energy gap, \(E_g\), is the vertical distance between the valence and conduction bands. It represents the minimum amount of energy needed to excite an electron. The Fermi energy level represents the energy level at which the probability of finding an electron is 0.5. At \(T = 0\) K, it is at the middle of the energy gap. In the figure, it is shown as a dotted line. Qualitatively, it represents the amount of energy needed to remove the next electron. No electrons have exactly that energy because there are no allowed states in the gap. For a doped semiconductor, a semiconductor with crystalline defects, or a semiconductor not at absolute zero temperature, the Fermi level is near but not quite at the middle of the gap.
The right part of the figure shows the same energy level diagram labeled using terms more commonly used by chemists to describe isolated atoms than by physicists to describe solid semiconductors. Chemists sometimes use the term highest occupied energy level instead of valence band. This term is most often used to refer to energy levels of isolated atoms or molecules because some authors reserve the term band for an energy level shared between neighboring atoms. Similarly, chemists sometimes use the term lowest unoccupied energy level in place of conduction band. As discussed below in Secs. 9.2.3 and 9.2.4, the term chemical potential \(\mu_{chem}\) is used in place of Fermi energy level \(E_f\), and the energy gap \(E_g\) may be called twice the chemical hardness.
Mulliken Electronegativity
One measure that chemists use to describe the ability of charges to flow is electronegativity, and this term has multiple definitions in the literature. One definition is by Mulliken in 1934 [130], and this measure will be referred to as the Mulliken electronegativity. Mulliken approximated the energy in a chemical bond by averaging the ionization energy \(I_{ioniz}\) and the electron affnity \(A_{af\,f}\). Mulliken electronegativity is defined
\[\chi_{Mulliken} = \frac{I_{ioniz} + A_{af\,f}}{2}. \label{9.2.1} \]
Ionization energy is the energy needed to remove an electron from an atom or ion, and electron affinity is the energy change when an electron is added to an atom or ion [12]. All of these quantities, \(\chi_{Mulliken}\), \(I_{ioniz}\), and \(A_{af\,f}\), are measured in the SI units of \(\frac{J}{atom}\) or occasionally in other units like \(\frac{eV}{atom}\) or \(\frac{kJ}{mol}\).
This definition is simpler than other definitions of electronegativity, and reference [131] calls this an "operational and approximate" definition. It is useful because it involves strength of chemical bonds, and we can relate it to the measures used by semiconductor researchers. Qualitatively, ionization energy is represented by the energy needed to rip off an electron. In Fig. \(\PageIndex{1}\), it is the vertical distance from the valence band or highest occupied state to the ground state at the top of the figure. Sometimes chemists call this amount of energy the work function instead [60, ch. 6] [108]. In Fig. \(\PageIndex{1}\), the electron affinity is represented by the vertical distance from the conduction band or lowest unoccupied state to the ground state at the top of the figure. The magnitude of the Mulliken electronegativity is the average of these two energies, so it is the magnitude of the Fermi energy at \(T = 0\) K. By convention, it has the opposite sign.
\[\chi_{Mulliken} = \left. -E_f \right|_{T=0}K \nonumber \]
Fundamentally, electrical engineering is the study of flow of charges. Chemistry is the study of the strength of chemical bonds. The electrical conductivity of a material is high when the chemical bonds holding that material together are easily broken so that many free charges can flow. The electrical conductivity of a material is low when chemical bonds holding atoms together require lots of energy to break. Electronegativity is a measure of the energy required to break chemical bonds, so fundamentally, it tells us similar information to electrical conductivity.
Chemical Potential and Electronegativity
Another way of defining electronegativity follows the definition introduced by Pritchard in 1956 [132]. This definition is one of the more common ones, and it is used by both chemists [131] [133] and by other scientists [2, p. 124.]. The electronegativity of an atom is defined as
\[\chi = \left. - \left( \frac{\partial \mathbb{U}}{\partial N} \right) \right|_{V,S,} \label{9.2.3} \]
where \(\mathbb{U}\) is the internal energy relative to a neutral atom and \(N\) represents the number of electrons around the atom. An atom is composed of a charged nucleus and charged electrons moving around the nucleus, so there is an electric field, and hence an electrical potential \(V\) in volts, around an atom. This potential significantly depends on the number of electrons around the atom. Also, when the atom is at a temperature above absolute zero, the electrons and nuclei are in motion, so the atom has some entropy \(S\). Electronegativity involves \(\frac{\partial \mathbb{U}}{\partial N}\) at constant electrical potential and entropy. It applies whether the atoms are part of a solid, liquid, or gas.
The chemical potential \(\mu_{chem}\) is defined as the negative of this electronegativity.
\[\mu_{chem} = -\chi \nonumber \]
In SI units, both chemical potential and electronegativity are measured in \(\frac{J}{atom}\), but sometimes they are also expressed in \(\frac{eV}{atom}\) or \(\frac{kJ}{mol}\). As if the three names, chemical potential, negative of the electronegativity, and Fermi energy level, weren't enough, this quantity is also known as the partial molar free energy [60, p. 145].
Electronegativity is used to describe a collection of atoms, molecules, or ions all of the same ionization state [131]. Less energy is required to rip the first electron off an atom than the second or third electron. The definition of electronegativity is specific to potential \(V\), in volts, due to the nucleus and electrons around an atom. For example, we can talk about the electronegativity, energy required to rip off the electron, of a neutral magnesium atom. We can also talk about the electronegativity, energy required to rip off an electron, from a Mg\(^+\) ion. The electric field, and hence potential \(V\), around a neutral Mg atom and the electric field, and hence potential \(V\), around a magnesium ion Mg\(^+\) are necessarily different because of the number of electrons present. The energies required to rip off the next electron from these atoms will also necessarily be different. So, electronegativity of a material always refers to a specific ionization state. Electronegativity incorporates both the energy required or gained by ripping off an electron and the energy required or gained by acquiring an electron. Qualitatively, it is the average of the ionization energy required to rip off an electron and the electron affinity released when an electron is captured. In the case of the Mg atom from the example above, the energy gained by releasing an electron is the significant term, but that is not always the case.
In most energy conversion devices, and most chemical reactions, we are interested in only the valence electrons. So, even if an atom has dozens of electrons around it and the energy to rip off each electron is different, we are just interested in the first few valence electrons. We will see that batteries and fuel cells involve energy stored in chemical bonds. Only the valence electrons are involved in the reactions of batteries and fuel cells, so in studying batteries and fuel cells, we are most interested in the electronegativity of neutral or singly ionized atoms.
Equation \ref{9.2.3} defines electronegativity as the energy required to rip off the next electron from the atom. Again consider Fig. \(\PageIndex{1}\). The energy level known as the valence band to semiconductor physicists and the highest occupied state to chemists is filled with electrons. The next highest band, called either the conduction band by semiconductor physicists or lowest unoccupied state by chemists, is not filled with electrons. The electronegativity according to this definition is the energy required to rip off the next electron. On average, it is again graphically represented by the Fermi level.
Both electronegativity defined by Equation \ref{9.2.3} and Mulliken electronegativity defined by Equation \ref{9.2.1} have the same units. However, multiple other definitions of electronegativity can be found in the literature. One of the oldest definitions is due to Pauling in 1932 [134], and that definition is measured instead in the units of square root of joules on a relative scale. Reference [135] expanded on Pauling's definition to show variation with ionization state and atom radius. Reference [133] also contains a different definition of electronegativity also with its own units.
Chemical Hardness
Chemists sometimes use the term hardness when semiconductor physicists would use the term half the energy gap. Chemical hardness has nothing to do with mechanical hardness. As with electronegativity, there are multiple related definitions of hardness. The Mulliken hardness is defined as [131]
\[\text{Mulliken hardness} = \frac{I_{ioniz} − A_{af\,f}}{2}. \nonumber \]
A more careful definition of chemical hardness is [131] [136, p. 93]
\[\text{hardness} = \left. \frac{1}{2} \left( \frac{\partial \mu_{chem}}{\partial N} \right) \right|_{V,S,}. \nonumber \]
It is half the change in chemical potential for the next electron, and qualitatively it is represented by half the energy gap. As with electronegativity, it is specified for a given potential in volts around the atom and a given entropy. Liquids may be classified as hard or soft. Hard acids and hard bases have large energy gaps, so they are electrical insulators. Soft acids and soft bases have small energy gaps, so they are electrical conductors. No additional variable will be introduced for hardness because this quantity can be represented by half the energy gap, \(\frac{E_g}{2}\).
Redox Potential
Redox (from REDuction-OXidation) potential \(V_{rp}\) is yet another measure used by chemists to describe the ability of electrons to be ripped off their atoms and flow in the presence of an applied voltage, nearby chemical, optical field, or other energy source. As defined above, the process of ripping off electrons is called oxidation. The process of gaining electrons is called reduction. Together, they form redox reactions. Instead of being measured in joules like electronegativity, it is measured in volts where a volt is a joule per coulomb. Redox potential represents the energy stored in a chemical bond per unit charge. It is more often used by experimentalists than theorists, and it is often used to describe solids instead of liquids. Redox potential is a macroscopic property, describing a larger piece of material as opposed to describing just an individual atom. It is also sometimes called oxidation reduction potential or the standard electrode potential [137]. It is a relative measure of the ability of a substance to lose an electron. A list of redox potentials can be found in references [60, p. 158] and [137]. There are different ways of defining redox potential in the literature. The definitions vary in their choice of a ground reference voltage, and they vary in their sign conventions. American and European researchers tend to use different definitions.
Redox potential is measured on a relative scale. To measure redox potential [138], electrodes are put in the system being studied. A potential is applied to balance the internal voltage. By measuring this externally applied voltage, the potential of an electrode is determined with respect to a reference electrode. Often, the potential of a platinum electrode is used as a reference and said to have zero volts at standard conditions of \(T = 25 ^{\circ}C\) and \(\mathbb{P} = 1\) atm. The reaction at the platinum electrode is
\[\text{H}_2 \rightarrow 2\text{H}^+ + 2e^-. \nonumber \]
pH
pH is a unitless measure of the likelihood that a water molecule is bonded or has been ionized in a liquid solution. It is used to classify liquids as acidic or basic. When discussing pH, we assume the material under test is a liquid solution at a temperature of \(25 ^{\circ}C\) and a pressure of 1 atm [12] [81]. A liquid solution is a mixture of water and another material called a solute. More specifically, pH is defined as
\[\text{pH} = \log_{10} \left( \frac{a}{[\text{H}^+]} \right). \nonumber \]
The quantity \([\text{H}^+]\) is the amount concentration of hydrogen ions in the units of mol L [68, p. 39].
\[[\text{H}^+] = \frac{\text{concentration H}^+ \text{ions, mol}}{\text{volume of solution in L}} \nonumber \]
This quantity was formerly called molarity or molar concentration, but these terms are no longer recommended for use [68, p. 39]. pH is a measure often used by experimentalists.
The concept of pH is fundamentally related to the flow of charges, a concept which is very important to electrical engineers. Water is composed of H\(_2\)O molecules. In pure water, some of these molecules fall apart, ionizing into H\(^+\) ions (protons) and OH\(^-\) ions. However, most of the molecules remain intact. If some solutes are mixed with the water, more of the H\(_2\)O molecules will ionize than in pure water. For example, carbon dioxide will bond with OH\(^-\) ions forming carbonic acid HCO\(_3^-\) causing an increase in H\(^+\) ions. Since pH is the negative log of H\(^+\) ion activity, increasing H\(^+\) ion concentration is equivalent to a pH decrease. If ammonia, NH\(_3\), is added to water, NH\(_4^{+}\) is formed, and the number of OH\(^-\) ions increases resulting in an increase in pH. Since water is a liquid, both these positive and negative ion charge carriers can move about relatively easily. If an external voltage is applied across the liquid, ions will flow. Electrical conductivity will be higher in a liquid with more ions present than in liquids with fewer ions present.
As an example, consider what happens when neutral sodium atoms are added to water. (For obvious reasons, don't try this at home [139].) It is energetically favorable for the sodium atoms to ionize to Na\(^+\) giving up an electron. In the process, some more water molecules ionize, and some H\(^+\) ions become neutral H atoms.
\[\mathrm{Na}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Na}^{+}+\mathrm{H}+\mathrm{OH}^{-} \nonumber \]
By adding the solute sodium, the solution has fewer H\(^+\) ions. Consider what happens when neutral chlorine atoms are added to water. It is energetically favorable for a chlorine atom to acquire an electron to a form Cl\(^-\) ion.
\[\mathrm{Cl}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Cl}^{-}+\mathrm{H}^{+}+\mathrm{OH} \nonumber \]
By adding the solute chlorine, the solution has more H\(^+\) ions. While these examples involve adding neutral atoms, the concept of pH applies to solutes which are molecules too.
Solutions with pH less than 7 are called acidic. If a solution has a high concentration of H\(^+\) ions, it will have a low pH and be acidic. In strongly acidic solutions, molecules of the solute rip apart many water molecules, so lots of ions are present. Solutions with pH greater than 7 are called alkaline or basic. If a solution has a low concentration of H\(^+\) ions, and hence a high concentration of OH\(^-\) ions, it will have a high pH and be alkaline. In strongly alkaline solutions, molecules of the solute rip apart many water molecules, so again lots of ions are present. Neutral solutions have a pH near 7, and some neutral solutions may be electrical insulators. Solutions with a pH much below or much above 7 necessarily have many ions present, and they are good electrical conductors.
As an example, let's find the pH of a solution with \(10^{15}\) ions of H\(^+\) in 1 L of water.
\[\left[\mathrm{H}^{+}\right]=\frac{10^{15} \text { ions }}{1 \mathrm{L}} \cdot \frac{1}{6.022 \cdot 10^{23} \frac{\mathrm{ions}}{\mathrm{mol}}}=1.66 \cdot 10^{-9} \frac{\mathrm{mol}}{\mathrm{L}} \nonumber \]
\[\mathrm{pH}=\log \left(\frac{1}{1.66 \cdot 10^{-9}}\right) \approx 9 \nonumber \]
Notice that the exponent of \([\text{H}^+]\) is -9, and the pH is 9. Qualitatively, the pH tells us the negative of the order of magnitude of the amount concentration of hydrogen ions. The solution in this example is alkaline.
As a related example, let's find the pH of a solution with \(10^{20}\) ions of H\(^+\) in 1 L of water.
\[\left[\mathrm{H}^{+}\right]=\frac{10^{20} \text { ions }}{1 \mathrm{L}} \cdot \frac{1}{6.022 \cdot 10^{23} \frac{\mathrm{ions}}{\mathrm{mol}}}=1.66 \cdot 10^{-4} \frac{\mathrm{mol}}{\mathrm{L}} \nonumber \]
\[\mathrm{pH}=\log \left(\frac{1}{1.66 \cdot 10^{-4}}\right) \approx 4 \nonumber \]
This example has more hydrogen ions in the solution, so it is more acidic. The pH of 4 tells us that the solution has approximately \(10^{-4} \frac{mol}{L}\) of hydrogen ions.
How many hydrogen ions are found in a 1 L solution with a pH of 7?
\[7=\log \left(\frac{1}{\left[\mathrm{H}^{+}\right]}\right) \nonumber \]
\[\left[\mathrm{H}^{+}\right]=10^{-7} \frac{\mathrm{ions}}{\mathrm{mol}} \nonumber \]
\[10^{-7} \frac{\mathrm{mol}}{\mathrm{L}} \cdot 1 \mathrm{L} \cdot 6.022 \cdot 10^{23} \frac{\mathrm{ions}}{\mathrm{mol}}=6.022 \cdot 10^{16} \mathrm{ions} \mathrm{H}^{+} \nonumber \]
A neutral solution, with a pH of 7, still contains H\(^+\) ions.


