5: Flows and Cycles of Nutrients
- Page ID
- 42028
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
After completing this chapter, you will be able to
- Explain what nutrients are and give examples.
- Discuss the concept of nutrient cycling and describe important compartments and fluxes.
- Describe factors that affect the development of major soil types.
- Describe the cycles of carbon, nitrogen, phosphorus, and sulphur.
Nutrients
Nutrients are any chemicals that are needed for the proper functioning of organisms. We can distinguish two basic types of nutrients: (1) inorganic chemicals that autotrophic organisms require for photosynthesis and metabolism, and (2) organic compounds ingested as food by heterotrophic organisms. This chapter deals with the inorganic nutrients.
Plants absorb a wide range of inorganic nutrients from their environment, typically as simple compounds. For example, most plants obtain their carbon as gaseous carbon dioxide (CO2) from the atmosphere, their nitrogen as the ions (charged molecules) nitrate (NO3–) or ammonium (NH4+), their phosphorus as phosphate (PO43–), and their calcium and magnesium as simple ions (Ca2+ and Mg2+). The ions are obtained in dissolved form in soil water absorbed by plant roots. Plants utilize these various nutrients in photosynthesis and other metabolic processes to manufacture all of the biochemicals they need for growth and reproduction.
Some inorganic nutrients, referred to as macronutrients, are needed by plants in relatively large quantities. These are carbon, oxygen, hydrogen, nitrogen, phosphorus, potassium, calcium, magnesium, and sulphur. Carbon and oxygen are required in the largest amounts because carbon typically comprises about 50% of the dry weight of plant biomass and oxygen somewhat less. Hydrogen accounts for about 6% of dry plant biomass, while nitrogen and potassium occur in concentrations of 1-2% and those of calcium, phosphorus, magnesium, and sulphur are 0.1-0.5%. Micronutrients are needed in much smaller amounts, and they include boron, chlorine, copper, iron, manganese, molybdenum, and zinc. Each of these accounts for less than 0.01% of plant biomass and as little as a few parts per million (ppm, or 10–6; 1 ppm is equivalent to 0.0001%; see Appendix A).

Heterotrophs obtain the nutrients they require from the food they eat, which may be plant biomass (in the case of a herbivore), other heterotrophs (carnivore), or both (omnivore). The ingested biomass contains nutrients in various organically bound forms. Animals digest the organic forms of nutrients in their gut and assimilate them as simple organic or inorganic compounds, which they use to synthesize their own necessary biochemicals through various metabolic processes.
Nutrient Flows and Cycles
Although Earth gains small amounts of material through meteorite impacts, these extraterrestrial inputs are insignificant in comparison with the mass of the planet. Essentially, at the global level, Earth is an isolated system in terms of matter. As a consequence of this fact, nutrients and other materials “cycle” within and between ecosystems. In contrast, energy always “flows through” ecosystems and the biosphere (Chapter 4). Nutrient cycling refers to the transfers, chemical transformations, and recycling of nutrients in ecosystems. A nutrient budget is a quantitative (numerical) estimate of the rates of nutrient input and output to and from an ecosystem, as well as the amounts present and transferred within the system.
The major elements of a nutrient cycle are shown in Figure 5.1. The outer boundary of the diagram defines the limits of an ecosystem. (It could even represent the entire biosphere, in which case there would be no inputs to or outputs from the system.) In ecological studies, the system is often defined as a particular landscape, lake, or watershed (a terrestrial basin from which water drains into a stream or lake). Each of these systems has inputs and outputs of nutrients, the rates of which can be measured.
The boxes within the boundary represent compartments, each of which stores a quantity of material. Compartment sizes are typically expressed in units of mass per unit of surface area. Examples of such units are kilograms per hectare (kg/ha) or tonnes per hectare (t/ha). In aquatic studies, compartment sizes may be expressed per unit of water volume (such as g/m3). The arrows in the diagram represent fluxes, or transfers of material between compartments. Fluxes are rate functions, and are measured in terms of mass per area per time (e.g., kg/ha-yr).
The system can be divided into four major compartments:
- The atmosphere consists of gases and small concentrations of suspended particulates and water vapor.
- Rocks and soil consist of insoluble minerals that are not directly available for uptake by organisms.
- Available nutrients are present in chemical forms that are water soluble to some degree, so they can be absorbed by organisms from their environment and contribute to their mineral nutrition.
- The organic compartment consists of nutrients present within living and dead organic matter. This compartment can be divided into three functional groups: (a) living biomass of autotrophs such as plants, algae, and autotrophic bacteria, (b) living heterotrophs including herbivores, carnivores, omnivores, and detritivores, and (c) and all forms of dead organic matter.
The major transfers of material between compartments, or fluxes, are also shown in Figure 5.1. These are important transfer pathways within nutrient cycles. For instance, insoluble forms of nutrients in rocks and soil become available for uptake by organisms through various chemical transformations, such as weathering, that render the nutrients soluble in water. This is reversed by reactions that produce insoluble compounds from soluble ones. These latter reactions form secondary minerals such as carbonates (e.g. limestone, CaCO3, and dolomite, MgCO3), oxides of iron and aluminum (Fe2O3 and Al(OH)3), sulphides (e.g., iron sulphide, FeS2), and other compounds that are not directly available for biological uptake.
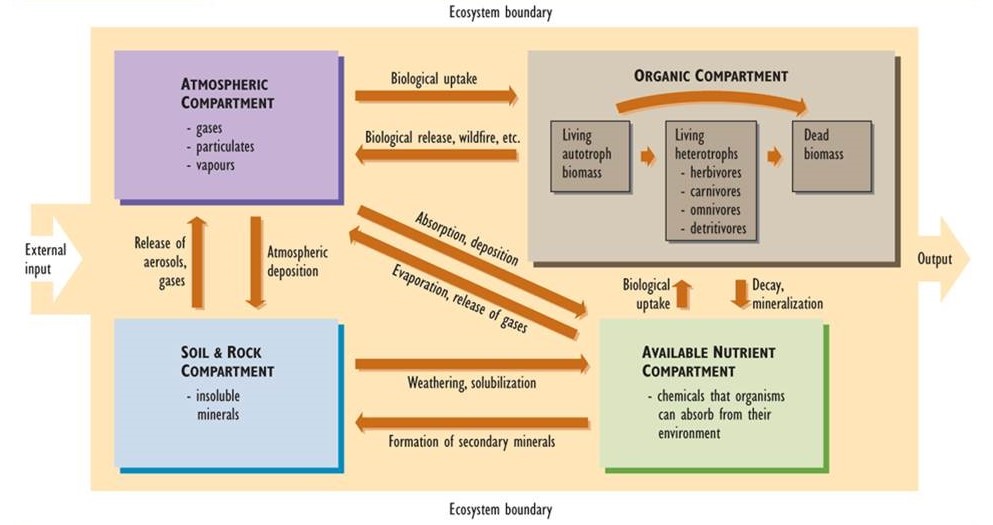
Other fluxes in nutrient cycles include the biological uptake of nutrients from the atmosphere or from the available pool in soil. For example, plant foliage assimilates carbon dioxide (CO2) from air, and roots absorb nitrate (NO3–) and ammonium (NH4+) ions dissolved in soil water. Plants then metabolically fix these nutrients into their growing biomass. The organic nutrients may then enter the food web and are eventually deposited as dead biomass. Organic nutrients in dead biomass are recycled through decay and mineralization, which regenerate the supply of available nutrients.
These concepts are examined in more detail in the following sections. Initially, we examine the soil ecosystem, which is where most nutrient cycling occurs within terrestrial habitats. We will then examine key aspects of the cycling of carbon, nitrogen, phosphorus, and sulphur.
The Soil Ecosystem
Soil is a complex and variable mixture of fragmented rock, organic matter, moisture, gases, and living organisms that covers almost all terrestrial landscapes. Soil provides mechanical support for growing, even for trees as tall as 100 m. Soil also stores water and nutrients for use by plants and provides habitat for the many organisms that are active in the decomposition of dead biomass and recycling of its nutrient content. Soil is a component of all terrestrial ecosystems, but it is also in itself a dynamic ecosystem.
Soil develops over long periods of time toward a mature condition. Fundamentally, soil is derived from a so-called parent material, which consists of rocks and minerals that occur within a metre or so of the surface. Parent materials in most of Canada were deposited through glacial processes, often as a complex mixture known as till, which contains rock fragments of various sizes and mineralogy. In some areas, however, the parent materials were deposited beneath immense inland lakes, usually in post-glacial times. Such places are typically flat and have uniform, fine-grained soils ranging in texture from clay to sand. (Clay particles have a diameter less than 0.002 mm, while silt ranges from 0.002 to 0.05 mm, sand from 0.05 to 2 mm, gravel from 2 to 20 mm, and coarse gravel and rubble are larger than 20 mm.) Figure 5.2 presents a textural classification of soil based on the percentage of clay-, silt-, and sand-sized particles.
Figure 5.2. A Textural Classification of Soils. The percentage composition of clay-, silt-, and sand-sized particles is used to classify soils into the 12 major types that are shown. Source: Modified from Foth (1990).
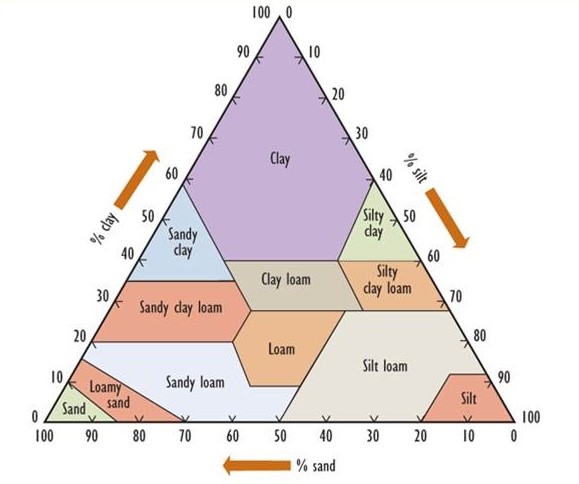
In other regions, parent materials known as loess are derived from silt that was transported by wind from other places. Because of their very small particle size, soil rich in clay has an enormous surface area, giving it important chemical properties such as the ability to bind many nutrient ions.
The characteristics of the parent material have an important influence on the type of soil that eventually develops. However, soil development is also profoundly affected by biological processes and climatic factors such as precipitation and temperature.
For example, water from precipitation dissolves certain minerals and carries the resulting ions downward. This process, known as leaching, modifies the chemistry and mineralogy of both the surface and deeper parts of the soil. In addition, inputs of litter (dead biomass) from plants increase the content of organic matter in soil. Fresh litter is a food substrate for many decomposer species of soil-dwelling animals, fungi, and bacteria. These organisms eventually oxidize the organic debris into carbon dioxide, water, and inorganic nutrients such as ammonium, although some material remaining as complex organic matter, known as humus. As soils develop, they assume a vertical stratification known as a soil profile, which has recognizable layers known as horizons. From the surface downward, the major horizons of a well-developed soil profile are as follows: 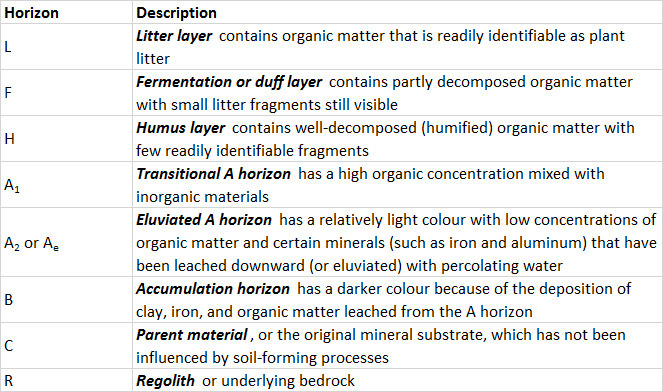
Soil that has been modified by human influences may be stratified differently. In cultivated land, for example, a homogeneous plough layer (Ap) of 15-20 cm develops at the surface. The plough layer is uniform in structure because it has been repeatedly mixed up for many years. In addition, the soil of agricultural land is often deficient in organic matter, compacted by the repeated passage of heavy machinery, and degraded in structure, nutrient concentration, and other qualities important to its ability to support crop productivity. These subjects are examined in more detail in Chapters 14 and 24.

Broadly speaking, soil within a particular kind of ecosystem, such as tundra, conifer forest, hardwood forest, or prairie, tends to develop in a distinctive way. Soils are classified by the ecological conditions under which they developed. The highest level of classification arranges soils into groups called orders, which can themselves be divided into more detailed assemblies. The most important soil orders in Canada are:

The Importance of Soil
The soil ecosystem is extremely important. Terrestrial plants obtain their water and much of the nutrients they need from the soil, absorbing them through their roots. Soil also provides habitat for a great diversity of animals and microorganisms that play a crucial role in litter decomposition and nutrient cycling.
Soil is economically important because it critically influences the kinds of agricultural crops that can be grown (this topic is examined in Chapter 14). Some of the most productive agricultural soils are alluvial deposits found along rivers and their deltas, where periodic flooding and silt deposition bring in abundant supplies of nutrients. As long as they are not too stony, chernozem and brunisol are also fertile and useful for agriculture. Much prairie agriculture is developed on chernozem soils, while much of the fertile agricultural land of southern Quebec and Ontario has brunisol types.
The Carbon Cycle
Carbon is one of the basic building blocks of life and the most abundant element in organisms, accounting for about half of typical dry biomass. Key aspects of the global carbon cycle are presented in Figure 5.3 (see also Chapter 17 and Figure 17.1). Gaseous carbon dioxide (CO2) is the most abundant form of carbon in the atmosphere, where it occurs in a concentration of about 400 ppm (0.04%), although methane (CH4, 1.8 ppm) is also significant.
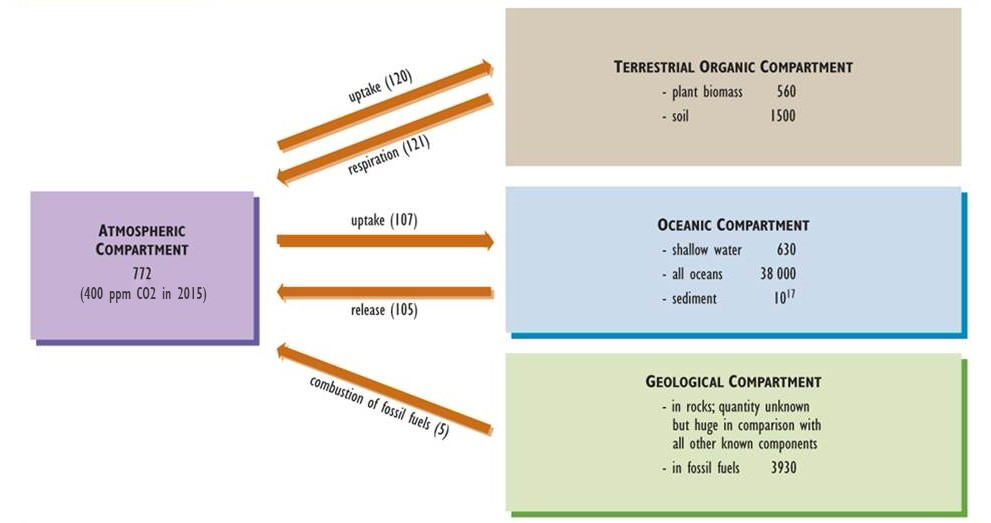
Atmospheric CO2 is a critical nutrient for photosynthetic organisms, such as plants and algae. Plants absorb this gas through tiny pores (called stomata) in their foliage, fix it into simple sugars, and then use the fixed energy to support their respiration and to achieve growth and reproduction. The biomass of autotrophs is available to be consumed by heterotrophs and passed through food webs. All organisms release CO2 to the atmosphere as a waste product of their respiratory metabolism.
CO2 is also the most common emission associated with the decomposition of dead organic matter. However, if this process occurs under anaerobic conditions (in which oxygen, O2, is not present), then both CO2 and CH4 are emitted. Because anaerobic decomposition is relatively inefficient, dead organic matter often accumulates in wetlands such as swamps and bogs, eventually forming peat. Under suitable geological conditions of deep burial, high pressure and temperature, and a lack of oxygen, peat and other organic materials may be slowly transformed into carbon-rich fossil fuels such as coal, petroleum, and natural gas (see Chapter 13).
Atmospheric CO2 also dissolves into oceanic water, forming the bicarbonate ion (HCO3–), which can be taken up and fixed by photosynthetic algae and bacteria, which are the base of the marine food web. Various marine organisms also use oceanic CO2 and HCO3– to manufacture their shells of calcium carbonate (CaCO3), an insoluble mineral that slowly accumulates in sediment and may eventually lithify into limestone (also CaCO3).
Over almost all of geological time, the amount of CO2 absorbed by the global biota from the atmosphere was similar to that released through respiration and decomposition. Consequently, the cycling of this nutrient can be viewed as a steady-state system. In modern times, however, anthropogenic emissions have changed the atmospheric carbon balance. Global emissions of CO2 and CH4 are now larger than the uptake of these gases, an imbalance that has resulted in increasing concentrations in the atmosphere. This phenomenon appears to be intensifying the greenhouse effect of Earth and resulting in global warming (see Chapter 17).
The Nitrogen Cycle
Nitrogen is another important nutrient for organisms, being an integral component of many biochemicals, including amino acids, proteins, and nucleic acids. Like the carbon cycle, that of nitrogen has an important atmospheric phase. However, unlike carbon, nitrogen is not a significant constituent of rocks and minerals. Consequently, the atmospheric reservoir plays a paramount role in the cycling of nitrogen (Figure 5.4).
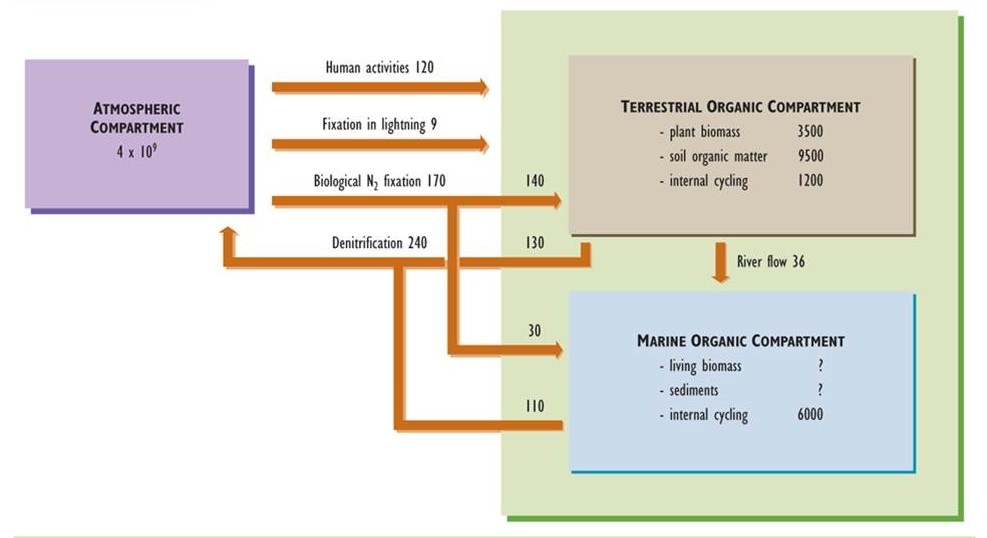
Virtually all nitrogen in the atmosphere occurs in the form of nitrogen gas (N2, sometimes referred to as dinitrogen), which is present in a concentration of 78%. Other gaseous forms of nitrogen are ammonia (NH3), nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O). These trace gases typically occur in atmospheric concentrations much less than 1 ppm, although there may be larger amounts close to sources of anthropogenic emissions (see Chapter 16). Nitrogen also occurs in trace particulates containing nitrate (NO3–) and ammonium (NH4+), such as ammonium nitrate (NH4NO3) and ammonium sulphate ((NH4)2SO4), both of which can be significant pollutants related to acid rain and haze (see Chapters 16 and 19).
Nitrogen occurs in many additional forms in terrestrial and aquatic environments. “Organic nitrogen” refers to the great variety of nitrogen-containing molecules in living and dead biomass. These chemicals range in character from simple amino acids, through proteins and nucleic acids, to large and complex molecules that are components of humified organic matter. Nitrogen in ecosystems also occurs in a small number of inorganic compounds, the most important of which are N2 and NH3 gases and the ions nitrate, nitrite (NO2–), and ammonium. The nitrogen cycle involves the transformation and cycling of the various organic and inorganic forms of nitrogen within ecosystems.
Nitrogen Fixation
Because the two nitrogen atoms in dinitrogen gas are held together by a strong triple bond, N2 is a highly unreactive compound. For this reason N2 can be directly used by only a few specialized organisms, even though it is extremely abundant in the environment. These nitrogen-fixing species, all of which are microorganisms, have the ability to metabolize N2 into NH3 gas, which can then be used for their nutrition. More importantly, the NH3 also becomes indirectly available to the great majority of autotrophic plants and microorganisms that cannot fix N2 themselves.
Biological nitrogen fixation is a critical process – most ecosystems depend on it to provide the nitrogen that sustains their primary productivity. In fact, because nitrogen is not an important constituent of rocks and soil minerals, N2 fixation is ultimately responsible for almost all of the organic nitrogen in the biomass of organisms and ecosystems throughout the biosphere. The only other significant sources of fixed nitrogen for ecosystems are the atmospheric deposition of nitrate and ammonium in precipitation and dustfall, and the uptake of NO and NO2 gases by plants. However, these are generally minor sources in comparison with biological N2 fixation.
The best known of the N2-fixing microorganisms are bacteria called Rhizobium, which live in specialized nodules on the roots of leguminous plants, such as peas and beans. Some non-legumes, such as alders, also live in a beneficial symbiosis (a mutualism; see Chapter 9) with N2-fixing microorganisms. So do most lichens, which are a mutualism between a fungus and an alga. Many other N2-fixing microbes are free-living in soil or water, such as cyanobacteria (blue-green bacteria).
Non-biological nitrogen fixation also occurs, for instance during a lightning event when atmospheric N2combines with O2 under conditions of great heat and pressure. Humans can also cause N2 to be fixed. For example, nitrogen fertilizer is manufactured by combining N2 with hydrogen gas (H2, which is manufactured from CH4, a fossil fuel) in the presence of iron catalysts to produce NH3. In addition, NO gas is formed in the internal combustion engines of vehicles, where N2 combines with O2 under conditions of high pressure and temperature. Large amounts of NO are emitted to the atmosphere in vehicle exhaust, contributing to air pollution (Chapter 16). Anthropogenic N2 fixation now amounts to about 120 million tonnes per year, about 83% of which is the manufacturing of fertilizer. This is a globally important component of the modern nitrogen cycle and is comparable in magnitude with non-human N2fixation (about 170 million tonnes per year).

Ammonification and Nitrification
After an organism dies, its organically bound nitrogen must be converted to inorganic forms; otherwise, the recycling of its fixed nitrogen would not be possible (Figure 5.5). The initial stage of this process is ammonification, in which the organic nitrogen of dead biomass is transformed to ammonia, which acquires a hydrogen ion (H+) to form ammonium (NH4+). As such, ammonification is a component of the complex process of decay, but one that is specific to the nitrogen cycle. Ammonification is carried out by a variety of microorganisms. The resulting ammonium is a suitable source of nutrition for many species of plants, particularly those that live in environments with acidic soil. Most plants, however, cannot utilize NH4+ effectively, and they require nitrate (NO3–) as their main source of nitrogen nutrition.
Nitrification is the process by which nitrate is synthesized from ammonium. The initial step is the conversion of NH4+ to nitrite (NO2–), a function carried out by bacteria known as Nitrosomonas. Once the nitrite is formed, it is rapidly oxidized to nitrate by Nitrobacter bacteria. Because Nitrosomonas and Nitrobacter are sensitive to acidity, nitrification does not occur in acidic soil or water. This is why plants growing in acidic habitats must be able to use ammonium as their source of nitrogen.
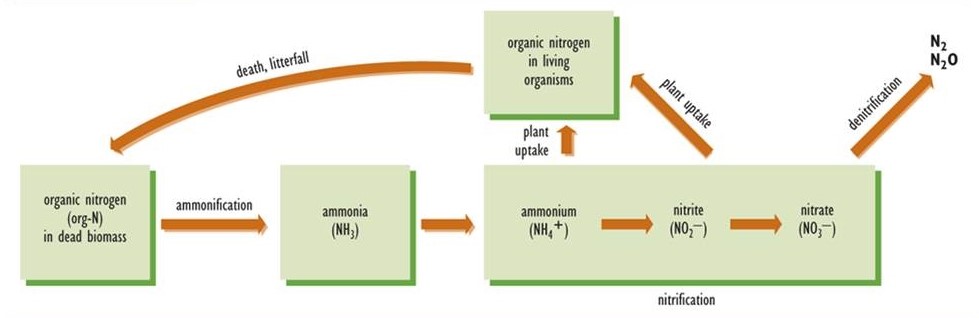
Denitrification
In denitrification, also performed by a wide variety of microbial species, nitrate is converted to either of the gases N2O or N2, which are released to the atmosphere. Denitrification occurs under anaerobic conditions, and its rate is greatest when there is a large concentration of nitrate, for example in fertilized agricultural land that is temporarily flooded. In some respects, denitrification can be considered a counter-balancing process to nitrogen fixation. In fact, global rates of nitrogen fixation and denitrification are in a rough balance, so the total amount of fixed nitrogen in the biosphere is not changing much over time.
The Phosphorus Cycle
Phosphorus is a key constituent of many biochemicals, including fats and lipids, nucleic acids such as the genetic materials DNA and RNA, and energy-carrying molecules such as ATP. However, phosphorus is required by organisms in much smaller quantities than nitrogen or carbon. Nevertheless, phosphorus is often in short supply and so it is a critical nutrient in many ecosystems, particularly in freshwater and agriculture.
In contrast to the carbon and nitrogen cycles, that of phosphorus does not have a significant atmospheric phase. Although phosphorus compounds do occur in the atmosphere, as trace quantities in particulates, the resulting inputs to ecosystems are small compared with the amounts available from soil minerals or from the addition of fertilizer to agricultural land. Phosphorus tends to move from the terrestrial landscape into surface waters and then eventually to the oceans, where it deposits to sediment that acts as a long-term sink. Although some phosphorus minerals in oceanic sediment are eventually recycled to the land by geological uplift associated with mountain building, this is an extremely slow process and is not meaningful in ecological time scales. Therefore, aspects of the global phosphorus cycle represent a flow-through system.
Nevertheless, certain processes do return some marine phosphorus to portions of the continental landscape. For example, some kinds of fish spend most of their life at sea but migrate up rivers to breed. When they are abundant, fish such as salmon import substantial quantities of organic phosphorus to the higher reaches of rivers, where it is decomposed to phosphate after the fish spawn and die. Fish-eating marine birds are also locally important in returning oceanic phosphorus to land through their excrement.
Soil is the principal source of phosphorus uptake for terrestrial vegetation. The phosphate ion (PO43–) is the most important form of plant-available phosphorus. Although phosphate ions typically occur in small concentrations in soil, they are constantly produced from slowly dissolving minerals such as calcium, magnesium, and iron phosphates (Ca3(PO4)2, Mg3(PO4)2, and FePO4). Phosphate is also produced by the microbial oxidation of organic phosphorus, a component of the more general process of decay. Water-soluble phosphate is quickly absorbed by microorganisms and by plant roots and used in the synthesis of a wide range of biochemicals.
Aquatic autotrophs also use phosphate as their principal source of phosphorus nutrition. In fact, phosphate is commonly the most important limiting factor to the productivity of freshwater ecosystems. This means that the primary productivity will increase if the system is fertilized with phosphate, but not if treated with sources of nitrogen or carbon (unless they first have sufficient PO43– added; see Chapter 20). Lakes and other aquatic ecosystems receive most of their phosphate supply through runoff from terrestrial parts of their watershed, and by the recycling of phosphorus from sediment and organic phosphorus suspended in the water column.
Humans are greatly affecting the global phosphorus cycle by mining it to manufacture fertilizer, and applying that material to agricultural land to increase its productivity. For some time, the major source of phosphorus fertilizers was guano, the dried excrement of marine birds. Guano is mined on islands, such as those off coastal Chile and Peru, where breeding colonies of seabirds are abundant and the climate is dry, allowing the guano to accumulate. During the twentieth century, however, deposits of sedimentary phosphate minerals were discovered in several places, such as southern Florida. Phosphorus had become geologically concentrated in sedimentary deposits in these places through the deposition of marine organisms over millions of years. These deposits are now being mined to supply mineral phosphorus used to manufacture agricultural fertilizer. However, when these easily exploitable mineral deposits become exhausted, phosphorus may turn out to be a limiting factor for agricultural production in the not-so-distant future.
About 50 million tonnes of phosphorus fertilizer are manufactured each year. This is a highly significant input to the global phosphorus cycle, in view of the estimate that about 200 million tonnes of phosphorus per year are absorbed naturally from soil by vegetation.
Image 5.4. Where colonial seabirds are abundant, their excrement (guano) can be mined as a source of phosphorus-rich fertilizer. This is a view of a large colony of fish-eating guanay cormorants (Phalacrocorax bougancillii) near Paracas off the coast of Peru. The dried guano is periodically scraped from the rocks and used for agricultural purposes. Source: B. Freedman.

Enviromental Issues 5.1. Too Much of a Good Thing – Pollution by Nutrients
Nutrients are essential to the healthy metabolism of organisms and to the proper functioning of ecosystems. Often, an increase in the supply of certain nutrients will enhance the productivity of wild and cultivated plants – this is the principle behind the use of fertilizer in agriculture. However, there are also cases in which an excessive supply of nutrients has caused important environmental problems.
Because the supply of available forms of nitrogen (particularly NO3– and NH4+) is often a limiting factor to agricultural productivity, these are generally the most abundant nutrients in fertilizer. However, the use of agricultural fertilizer can result in concentrations of NO3– in drinking water that are high enough to be toxic to humans, especially to infants (see Chapter 24). We also know that plants can take up gaseous NO and N2O from the atmosphere and use them as nutrients, along with NO3– and NH4+ from precipitation and soil water. Yet gaseous NO and N2O are air pollutants if they occur in high concentrations, especially in sunny environments where they are involved in the photochemical production of toxic ozone (see Chapter 16). Furthermore, large amounts of NO3– and NH4+ in rain and snow may contribute to acid rain (see Chapter 19).
There are other examples of environmental problems caused by excessive nutrients. For instance, CO2 is one of the most important plant nutrients because carbon comprises about half of plant biomass. But this critical nutrient occurs in a relatively small atmospheric concentration – only about 0.04%. However, the concentration of CO2 in the atmosphere has increased by about 45% during the past two centuries and it continues to amplify. This well-documented change is contributing to global warming, an important environmental problem (see Chapter 17).
Eutrophication, or an excessive productivity of waterbodies, is another environmental problem related to an excessive supply of nutrients. It is most often caused by an excess of PO43–, usually because of sewage dumping or runoff from fertilized agricultural land (see Chapter 20). Highly eutrophic lakes are degraded ecologically and may no longer be useful as a source of drinking water or for recreation.
Clearly, these examples show that there is a fine balance between chemicals serving as beneficial nutrients, or as damaging pollutants.
The Sulphur Cycle
Sulphur is a key constituent of certain amino acids, proteins, and other biochemicals. Sulphur is abundant in some minerals and rocks and has a significant presence in soil, water, and the atmosphere.
Atmospheric sulphur occurs in various compounds, some of which are important air pollutants (see Chapter 16). Sulphur dioxide (SO2), a gas, is emitted by volcanic eruptions and is also released by coal-fired power plants and metal smelters. SO2 is toxic to many plants at concentrations lower than 1 ppm. In some places, such as the Sudbury area, important ecological damage has been caused by this gas (Chapter 16).
In the atmosphere, SO2 becomes oxidized to the anion (negatively charged ion) sulphate (SO42–), which occurs as tiny particulates or is dissolved in suspended droplets of moisture. In this form, the negative charge of sulphate must be balanced by the positive charge of cations such as ammonium (NH4+), calcium (Ca2+), or hydrogen ion (H+, a key element of “acid rain”; see Chapter 19).
Hydrogen sulphide (H2S), which has a smell of rotten eggs, is emitted naturally from volcanoes and deep-sea vents. It is also released from habitats where organic sulphur compounds are being decomposed under anaerobic conditions, and from oxygen-poor aquatic systems where SO42– is being reduced to H2S. Dimethyl sulphide is another reduced-sulphur gas that is produced in the oceans and emitted to the atmosphere. In oxygen-rich environments, such as the atmosphere, H2S is oxidized to sulphate, as is dimethyl sulphide, but more slowly.
Most emissions of SO2 to the atmosphere are associated with human activities, but almost all H2S emissions are natural. An important exception is the emission of H2S from sour-gas wells and processing facilities, for example, in Alberta. Overall, the global emission of all sulphur-containing gases is equivalent to about 251 million tonnes of sulphur per year. About 41% of this emission is anthropogenic and the rest is natural (see Chapter 16).
Sulphur occurs in rocks and soils in a variety of mineral forms, the most important of which are sulphides, which occur as compounds with metals. Iron sulphides (such as FeS2, called pyrite when it occurs as cubic crystals) are the most common sulphide minerals, but all of the heavy metals (such as copper, lead, and nickel) can exist in this mineral form. Wherever metal sulphides become exposed to an oxygen-rich environment, the bacterium Thiobacillus thiooxidans oxidizes the mineral, generating sulphate as a product. This autotrophic bacterium uses energy from this chemical transformation to sustain its growth and reproduction. This kind of primary productivity is called chemosynthesis (in parallel with the photosynthesis of plants). In places where large amounts of sulphide are oxidized, high levels of acidity are associated with the sulphate product, a phenomenon referred to as acid-mine drainage (see Chapter 19).
Sulphur also occurs in a variety of organically bound forms in soil and water. These compounds include proteins and other sulphur-containing substances in dead organic matter. Soil microorganisms oxidize organic sulphur to sulphate, an ion that plants can use in their nutrition.
Plants satisfy their nutritional requirements for sulphur by assimilating its simple mineral compounds from the environment, mostly by absorbing sulphate dissolved in soil water, which is taken up by roots. In environments where the atmosphere is contaminated by SO2, plants can also absorb this gas through their foliage. However, too much absorption can be toxic to plants – there is a fine line between SO2 as a plant nutrient and as a poison.
Human activities have greatly influenced certain fluxes of the sulphur cycle. Important environmental damage has been caused by SO2 toxicity, acid rain, acid-mine drainage, and other sulphur-related problems. However, sulphur is also an important mineral commodity, with many industrial uses in manufacturing and as an agricultural fertilizer. Most commercial sulphur is obtained by cleaning “sour” natural gas (methane, CH4) of its H2S content and by removing SO2 from waste gases at metal smelters.
Conclusions
Nutrients are chemicals that are essential for the metabolism of organisms and ecosystems. If they are insufficient in quantity, then ecological productivity is less than it potentially could be. Nutrients can also be present in excess, in which case environmental damage may be caused by toxicity and other problems. Nutrients routinely cycle among inorganic and organic forms within ecosystems. Key aspects of nutrient cycles are illustrated by the carbon, nitrogen, phosphorus, and sulphur cycles.
Questions for Review
- What are the basic aspects of a nutrient cycle? In your answer, describe the roles of compartments and fluxes.
- How is soil formed from a parent material? Include the influences of physical and biological processes in your answer.
- What are the major kinds of soil? How do they differ?
- What are the key chemical transformations in the nitrogen cycle, and which ones are affected by human influences?
Questions for Discussion
- Compare and contrast key aspects of the cycling of carbon, nitrogen, phosphorus, and sulphur.
- The use of nitrogen and phosphorus fertilizers is crucial to modern agriculture, yet these materials are manufactured from non-renewable resources and may not be so readily available in the future. What would be the consequences for agricultural production if these fertilizers were to become more expensive and less available?
- How do your daily activities affect aspects of the carbon cycle?
- If soil becomes acidic, the process of nitrification may no longer occur. What are consequences of this change for the nutrition of plants?
Exploring Issues
- A sewage-treatment plant has applied for permission to dispose its nutrient-rich sludge onto nearby agricultural land. You have been asked to design a study that would examine the effects of the sludge on the cycling of nitrogen and phosphorus in the agroecosystem. What key response variables should be measured during the study? What experiments would you recommend for examining the potential effects of the sludge on nutrient cycling and crop productivity?
References Cited and Further Reading
Atlas, R.M. and R. Bartha. 1998. Microbial Ecology: Fundamentals and Applications. 4th ed. Benjamin/Cummings, Menlo Park, CA.
Blasing, T.J. and K. Smith. 2007. Recent Greenhouse Gas Concentrations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN. cdiac.ornl.gov/pns/current_ ghg.html
Botkin, D.B. and E.A. Keller. 2014. Environmental Science: Earth as a Living Planet. 9th ed. Wiley & Sons, New York, NY.
Brady, N.C. and R.R. Weil. 2007. The Nature and Properties of Soils. 14th ed. Prentice Hall, New York, NY.
Freedman, B., J. Hutchings, D. Gwynne, J. Smol, R. Suffling, R. Turkington, R. Walker, and D. Bazeley. 2014. Ecology: A Canadian Context. 2nd ed. Nelson Canada, Toronto, ON.
Hutzinger, O. (ed.) 1982. The Handbook of Environmental Chemistry. Springer-Verlag, New York. Likens, G.E., and F.H. Bormann. 1999. Biogeochemistry of a Forested Ecosystem. 2nd ed. Springer-Verlag, New York.
Margulis, L., C. Matthews, and A. Haselton. 2000. Environmental Evolution. 2nd ed. MIT Press, Cambridge, MA.
Plaster, E.J. 2002. Soil Science and Management. 3rd ed. Delmar Thomson Learning, Florence, KY.
Post, W.M., T. Peng, W.R. Emanual, A.W. King, V.H. Dale, and D.L. DeAngelis. 1990. The Global Carbon Cycle. American Scientist, 78: 310-26.
Schlesinger, W.H. 1997. Biogeochemistry: An Analysis of Global Change. 2nd ed. Academic Press, San Diego, CA.


