19: Acidification
- Page ID
- 42043
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
After completing this chapter, you will be able to:
- Describe the most important chemical ingredients of precipitation and explain which ones may cause acidity to develop.
- Outline the spatial patterns of acidic precipitation in North America, and identify factors influencing this distribution.
- Explain the difference between the wet and dry deposition of acidifying substances and how their rates vary.
- Describe how water chemistry is affected as precipitation interacts with vegetation and soil, and explain the implications for surface waters.
- Identify factors that make fresh waters vulnerable to acidification.
- Describe the effects of acidification on freshwater organisms.
- Discuss the roles of liming and fertilization in the reclamation of acidified lakes.
- Explain the importance of reducing emissions of sulphur and nitrogen gases to mitigating the acidification of surface waters.
Introduction
Acidification is a process that is characterized by increasing concentrations of hydrogen ions (H+) in soil or water. It can cause metals and their compounds to ionize, producing ions (such as Al3+) in concentrations high enough to be toxic to plants, animals, and microorganisms. Consequently, increasing acidification is usually interpreted as a degradation of environmental quality. Acidification is caused by many influences, both natural and anthropogenic, but the most widespread problems are associated with a phenomenon commonly referred to as acid rain.
Acid rain has been an important problem in parts of North America since at least the 1950s, but it did not become a high-profile issue until the early 1970s. This rather sudden attention resulted from the discovery that acid rain was a widespread problem in Western Europe, and the realization that the same conditions likely occurred in North America. This awareness stimulated research in Canada and the United States, which demonstrated that acid rain was causing a widespread acidification of lakes and streams, and possibly of soil. The acidification of aquatic ecosystems was resulting in important ecological damage, including the loss of many fish populations. Buildings and other materials were also being damaged because acidity erodes metals, paint, and some kinds of quarried stone.
Strictly speaking, the term “acid rain” refers only to acidic rainfall, which along with snowfall accounts for wet deposition. However, acidifying chemicals are also deposited from the atmosphere when it is not raining or snowing, through the dry deposition of certain gases and particulates. A suitable phrase to define this complex of processes is “the deposition of acidifying substances from the atmosphere”, or more simply, acidifying deposition. In this chapter we examine natural and anthropogenic causes of the acidification of ecosystems. We focus on the chemical qualities of acidic precipitation and dry deposition, their effects on terrestrial and aquatic ecosystems, and how acidification can be avoided or mitigated.
In Detail 19.1: Acids and Bases
An acid is defined as a substance that donates protons (hydrogen ions, H+) during a chemical reactions. An aqueous solution is acidic if its concentration of H+ is more than 1 × 10-7moles per litre. In contrast, a base (alkali) donates hydroxyl ions (OH–) in chemical reactions. A solution is basic if its concentration of OH– exceeds 1 × 10-7mol/L. (A mole is a fundamental unit that measures the amount of a substance and is equal to 6.02 × 1023 molecules, atoms, or ions. This number is known as Avogadro’s constant and it is derived from the number of carbon atoms contained in 12 g (1 mole) of carbon-12.)
Acids and bases react together to form water and a neutral salt. If equal numbers of moles of each are present, the solution has both zero acidity and zero alkalinity – the concentrations of H+ and OH–are both exactly 1 × 10-7mol/L. Such a solution is said to be neutral.
Because extremely wide ranges of H+ and OH– concentrations are encountered in nature and in laboratories, acidity is measured in logarithmic units, which are referred to as pH (an abbreviation for “potential of hydrogen”). pH is defined as –log10H+], or the negative logarithm to base 10 of the aqueous concentration of hydrogen ion, expressed in units of moles per litre. Acidic solutions have a pH less than 7.0, while alkaline solutions have a pH greater than 7.0. Note that a one-unit difference in pH implies a 10-fold difference in the concentration of hydrogen or hydroxyl ions. The scale illustrated below shows the pH of some commonly encountered substances.
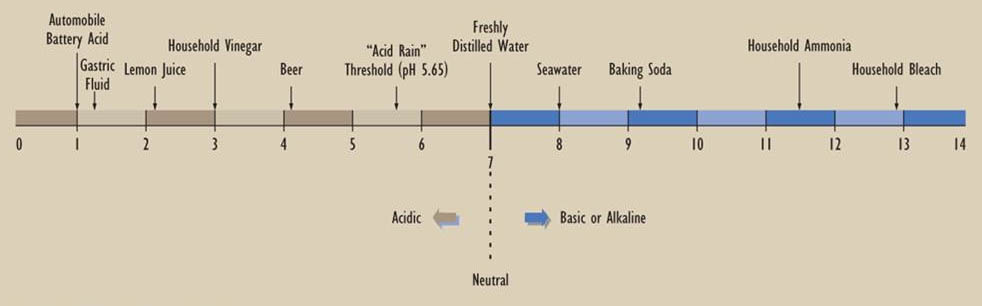
Chemistry of Precipitation
Scientists have adopted a functional definition of acidic precipitation as having a pH less than 5.65. This was chosen as the cut-off because at pH 5.65, an aqueous solution of carbonic acid (H2CO3) is in equilibrium with atmospheric CO2, as follows:
\[\ce{CO2 + H2O <=> H2CO3 <=> H^{+} + HCO3^{-} <=>⇌ 2H^{+} + CO3^{2-}}\]
This definition assumes that “non-acidic” precipitation is essentially distilled water, in which the acidity is determined only by the atmospheric concentration of CO2 and the amount of carbonic acid that subsequently develops. This is why the threshold below which precipitation is deemed “acidic” is set at the slightly acidic pH of 5.65, rather than at the strict zero-acidity pH of 7.0 (see In Detail 19.1).
It is, however, too simplistic to consider atmospheric moisture as consisting merely of distilled water in a pH equilibrium with gaseous CO2. Additional chemicals are also present in trace concentrations in precipitation. For example, on windy days, dust containing calcium and magnesium is blown into the atmosphere, and precipitation containing these elements may develop a pH higher than 5.65. This is especially true of agricultural and prairie landscapes, where the ground surface is often bare of plant cover and soil particles may be easily eroded into the atmosphere. In some other regions, a relatively high concentration of naturally occurring sulphate in the atmosphere may result in precipitation having a pH less than 5.65.
The most abundant cations (positively charged ions) in precipitation are hydrogen ion (H+), ammonium (NH4+), calcium (Ca2+), magnesium (Mg2+), and sodium (Na+). The most abundant anions (negatively charged ions) are sulphate (SO42-), chloride (Cl–), and nitrate (NO3-). Other ions are also present, but only in trace amounts that have little influence on the pH (see In Detail 19.2).
In Detail 19.2: Conservation of Electrochemical Neutrality
The principle of conservation of electrochemical neutrality states that in any electrically neutral solution (one that does not carry an electrical charge), the total number of positive charges associated with cations must equal the total number of negative charges of anions. For the purposes of calculating a charge balance, the concentrations of ions are measured in units known as equivalents. These are calculated as the molar concentration multiplied by the number of charges on the ion. (When dealing with precipitation or surface waters, microequivalents, or µeq, are generally the units reported.)
This principle is relevant to the acidification of water. The concentration of H+ can be determined as the difference in concentrations of the sum of all anion equivalents minus the sum of all cations other than H+. Therefore, if the total equivalents of anions exceed the total equivalents of cations other than hydrogen ion, then H+ must go into solution to balance the cation “deficit,” as follows:
H+ = (SO42- + NO3– + Cl–) – (Na+ + NH4+ + Ca2+ + Mg2+)
The above equation has proven to be useful in studies of acidic precipitation. Prior to about 1955, the measurement of pH was somewhat inaccurate. There were, however, reliable analyses of other important ions in surface waters and precipitation. In such cases, the equation can be used to calculate pre-1955 pH values, providing important data for studies of the historical pH in waters sensitive to acidification.
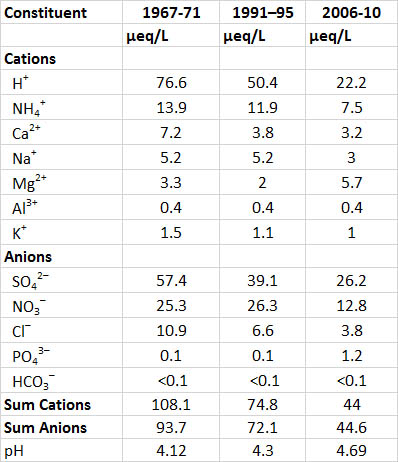
One of the longest-running records of precipitation chemistry is from a research site at Hubbard Brook, New Hampshire, in a region exposed to intense acidifying deposition. During 1967-1971, when acid rain was relatively severe, the average pH of precipitation at Hubbard Brook was 4.1. This level of acidity then relaxed somewhat to pH 4.9 in 1991-1995 because of decreased industrial emissions, particularly of the acid-forming gas SO2, and then even more so in 2009-2013 because of further decreases in SO2emissions (Table 19.1; see In Detail 19.2 for an explanation of equivalents). Sulphate and nitrate are the most important anions in precipitation, and from 1967-1971 they occurred accounted for 88% of the anion equivalents. During 2009-2013 these two still contributed 87% of the anion equivalents, although their total amounts were considerable smaller. These data suggest that most of the acidity in the precipitation occurs as dilute solutions of sulphuric and nitric acids. The precipitation events at Hubbard Brook that are most acidic are associated with storms that have passed over the large metropolitan regions of Boston, New York, and New Jersey. These areas have enormous emissions of SO2 and NOx, which are the precursor gases of much of the SO42- and NO3– in acidic precipitation.
Spatial Patterns
Acidic precipitation is a widespread phenomenon in eastern North America (Figure 19.1), Europe, eastern Asia, and elsewhere. In eastern North America prior to the mid-1950s, precipitation with pH below 4.6 affected only relatively local areas, mostly in southern Ontario, New York, Pennsylvania, and New England. Since then, however, this area has expanded considerably. At present, most of southeastern Canada and the eastern United States experiences acidic precipitation. It appears that the spatial pattern in North America existed before the 1950s, but the phenomenon has since become more widespread and its intensity has increased. One of the most important aspects of acidic precipitation is the vast size of the areas it affects.
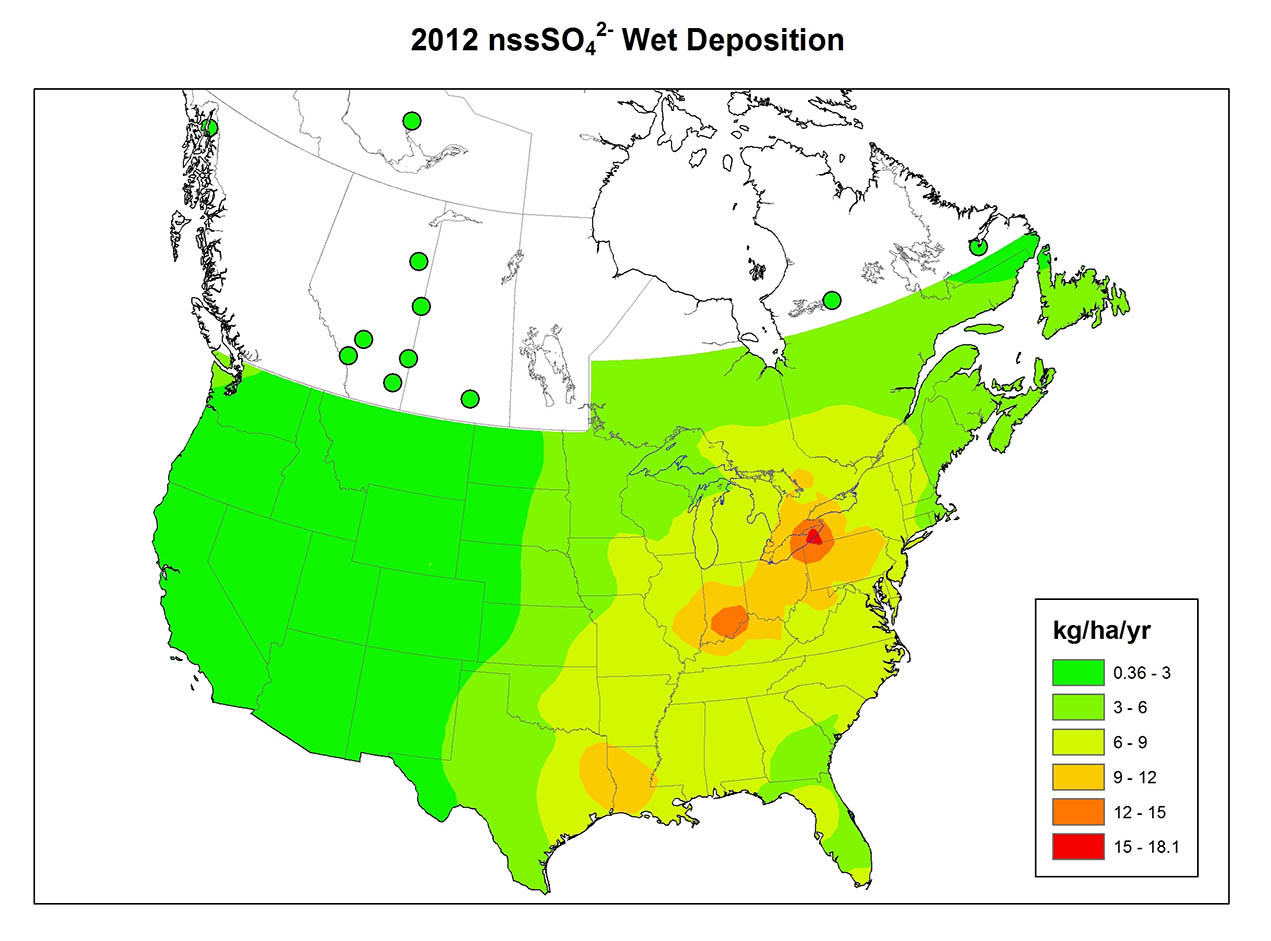
Precipitation chemistry varies greatly between regions (Figure 19.1). The variation reflects the patterns of emission of SO2 and NOx, the degree of oxidation of those gases to SO42- and NO3-, the prevailing direction travelled by polluted air masses, and the amount of acid-neutralizing dust in the atmosphere. Atmospheric dust is particularly important where vegetation cover is sparse, such as in agricultural regions where tiny soil particles are easily eroded into the atmosphere by strong winds blowing over bare fields. Unpaved roads are also an important source of atmospheric dust.
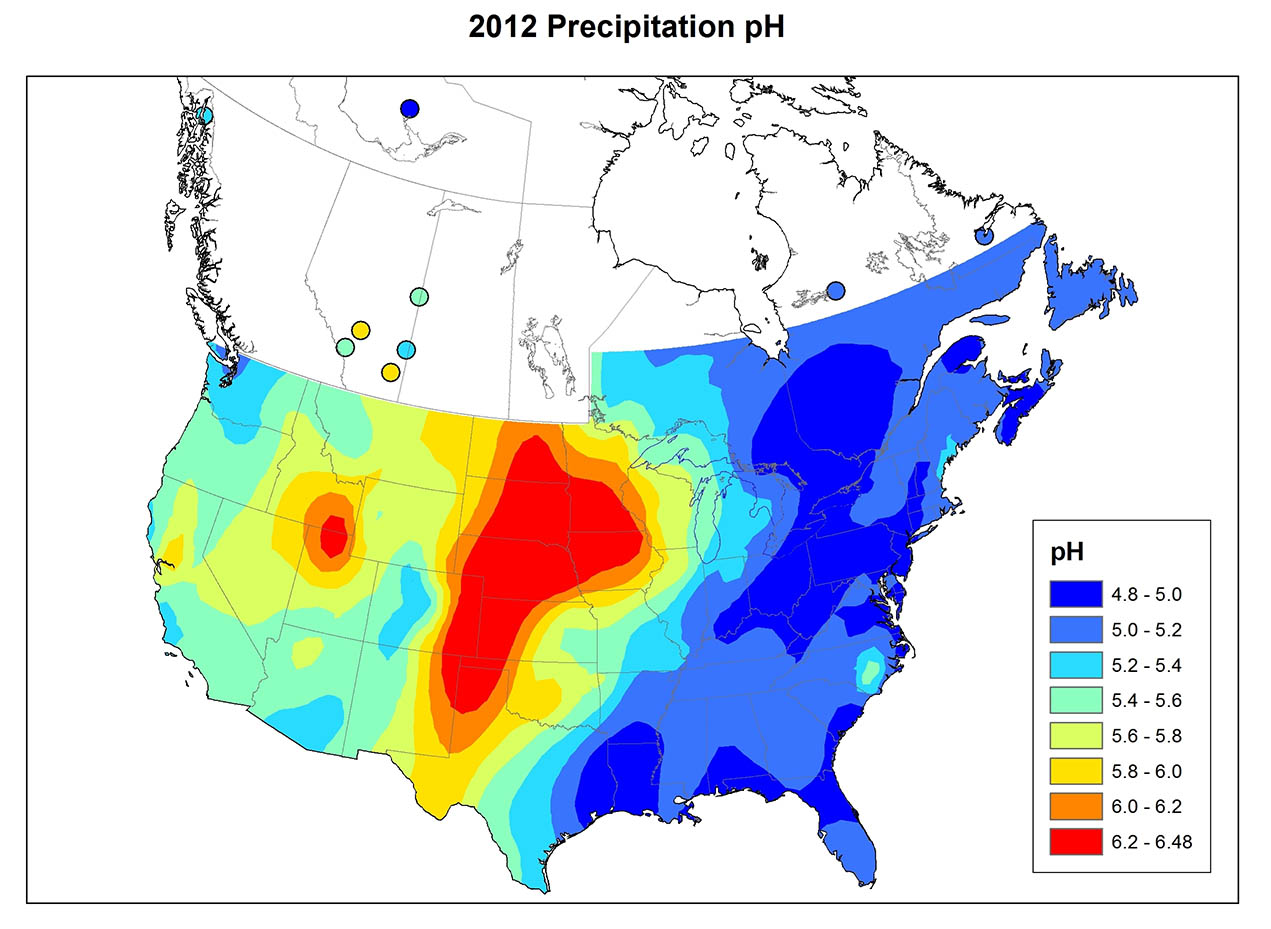
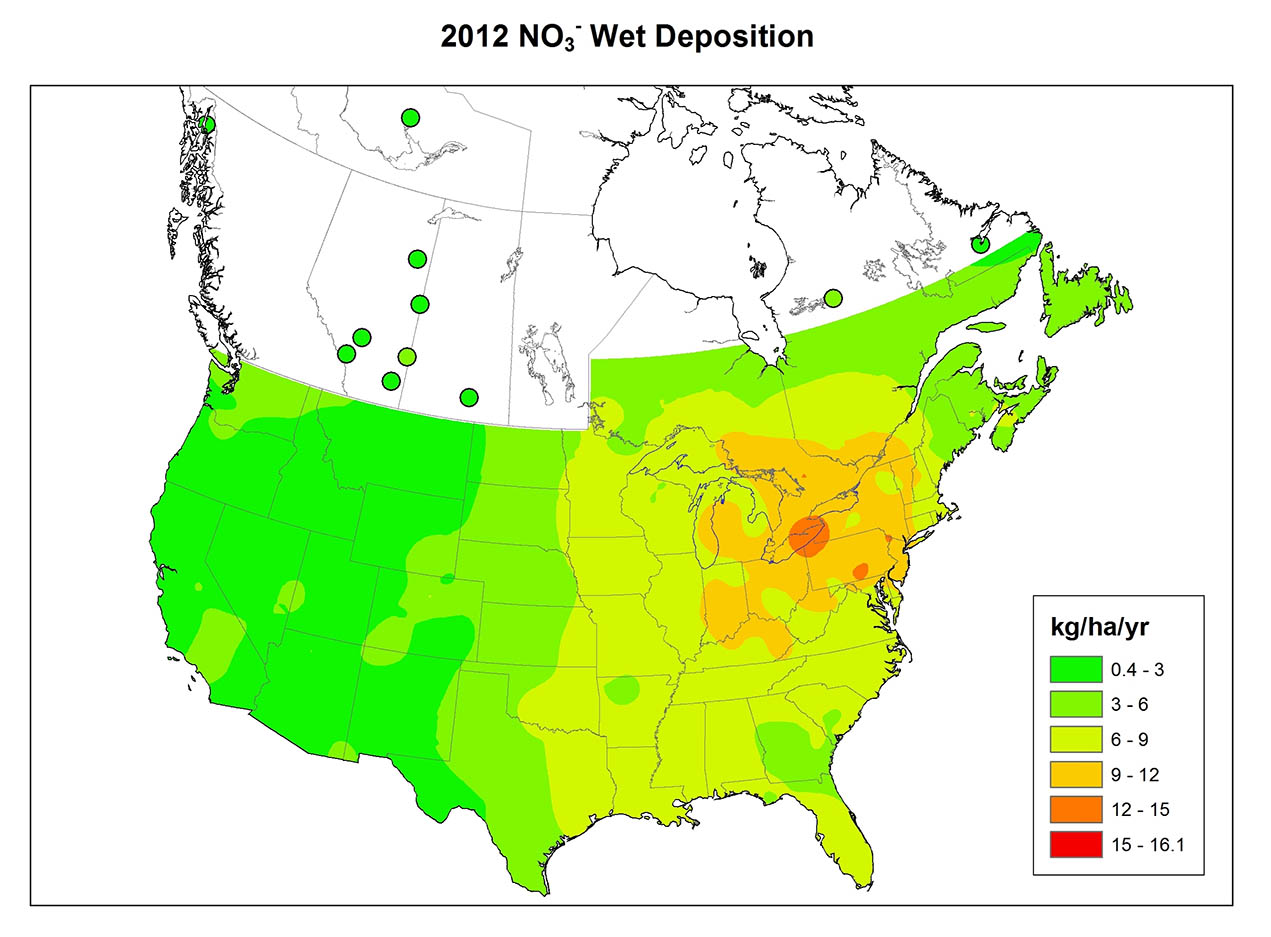
Information on the chemistry of precipitation at four widely separated Canadian sites is summarized in Table 19.2. Dorset is located in a rural area south-central Ontario, but not far north of the densely populated region of Greater Toronto (noted as “populated continental” in the table because it is an inland site close to large population centres to the south). The terrain is underlain by hard rocks of the Precambrian Shield, such as granite, gneiss, and quartzite, and the vegetation cover is mostly forest. The precipitation is highly acidic in the Dorset region, with an average pH of 4.1 and high concentrations of SO42- and NO3-, which suggests that the acidity is due mainly to dilute sulphuric and nitric acids. Much of the sulphate and nitrate in precipitation is derived from SO2 and NOx emitted by industries and automobiles to the south, which are then transported in the atmosphere before being deposited as acidifying deposition in the Dorset region.
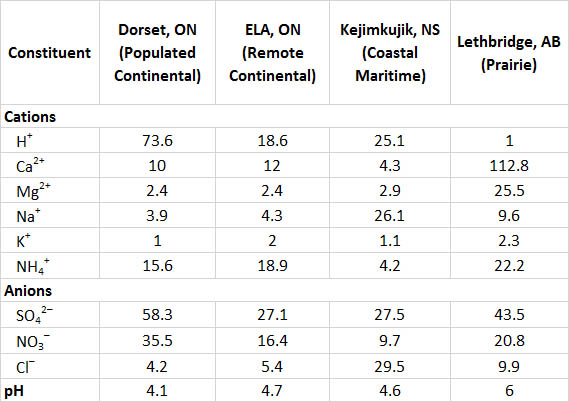
The ELA (Experimental Lakes Area) site is in a remote area of northwestern Ontario near Kenora (see Canadian Focus 20.1). Like Dorset, the ELA is in a largely forested landscape of Precambrian Shield, where bedrock and soil are composed of hard minerals such as granite and gneiss. However, the ELA site is much less influenced by air masses affected by anthropogenic emissions (labelled as “remote continental”), so its precipitation is not as acidic (average pH 4.7) than at Dorset and has less nitrate and sulphate.
The Kejimkujik site in western Nova Scotia is also underlain by hard granitic bedrock and the nearby terrain is forested. This site is distant from large sources of emissions of SO2 and NOx, but it often receives air masses that have passed over densely populated areas in the northeastern United States and eastern Canada. However, by the time the storm systems reach Kejimkujik, much of their acidic material has rained out, so the local precipitation is only moderately acidic (average pH 4.6). Kejimkujik is also influenced by oceanic weather systems, so its precipitation has high concentrations of sodium and chloride that are derived from sea spray. Oceanic salt water has a pH of about 8.0 because of the presence of chemicals such as bicarbonate, so marine aerosols have an acid-neutralizing influence on the precipitation in coastal regions.
Lethbridge is located in a landscape of mixed-grass prairie in southern Alberta. Precipitation there is non-acidic (average pH 6.0) because of the neutralizing influence of calcium- and magnesium-rich particulates that are relatively abundant in the atmosphere. These originate with dust blown up from agricultural fields and roads. This dust also accounts for the abundant Ca2+ and Mg2+ in the precipitation.
Rapid changes in precipitation chemistry may occur at the border between a forested landscape and areas dominated by prairie or agricultural land. A study in southern Ontario examined precipitation at eight places in a forested area with thin soil and Precambrian bedrock, and at three sites just south of the Shield in agricultural terrain with calcium-rich soil (Dillon et al., 1977). The average pH of precipitation among the Shield sites was 4.1-4.2, while at sites to the south it was 4.8-5.8. Precipitation was less acidic in the agricultural area because of the local neutralizing influence of dust blown up from fields and roads.

Another important characteristic of acidic precipitation is that, unlike SO2 and metal particulates, its intensity does not increase closer to large point sources of emissions, such as a coal-fired power plant or a smelter. For instance, precipitation is no more acidic close to the superstack at Sudbury than in the larger region, yet that is Canada’s largest point sources of SO2. Moreover, when that smelter was closed by a strike in 1979, the acidity of local precipitation did not change – it averaged pH 4.49 during the seven-month strike, compared with pH 4.52 during the prior seven months when there were large SO2 emissions (Scheider et al., 1980).
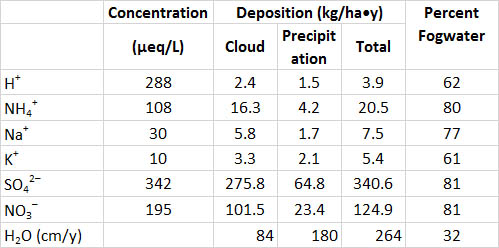
Fog moisture may also be quite acidic in eastern North America and elsewhere. Fogwater collected at high elevations and at coastal locations is commonly more acidic than pH 4.0, and it can be as acidic as pH 2.5-3.0. At forested sites where fog is a common occurrence, large amounts of acidity and other chemicals are filtered out of the atmosphere by trees. This occurs because tiny suspended water droplets coalesce on the surface of foliage and bark as fog passes through a forest canopy, a process that removes much of the fogwater from the atmosphere. This phenomenon is illustrated in Table 19.3 for a conifer forest on a mountain that often experiences foggy conditions. The total input of atmospheric moisture to that forest was 264 cm/year, with rain and snow accounting for 68% and fogwater 32% (fog was present 40% of the time). However, the concentrations of many chemicals are much higher in fogwater than in precipitation – because the suspended water droplets are tiny, the dissolved chemicals are less “diluted” by their aqueous matrix. As a result, the rates of deposition of dissolved substances to the forest can be higher than from precipitation. At this particular location, fog water accounted for 62% of the H+ deposition and 81% of the inputs of SO42- and NO3-.
Transboundary Air Pollution
Acidifying substances and their gaseous precursors are often transported over long distances in the atmosphere, far from their sources of emission. The acidifying chemicals do not respect political boundaries, so emissions occurring in one country can degrade ecosystems and valuable resources in other countries. This transboundary context has helped to focus the attention of governments on the problem of acidifying deposition from the atmosphere.
In Western Europe, for example, Scandinavians justifiably argued that most of the acidifying deposition that has affected extensive regions of their landscape has resulted from emissions of SO2 and NOx in Germany and England. This international European context was the first well-demonstrated case of so-called LRTAP, an acronym for the long-range transport of atmospheric pollutants.
Similar transboundary circumstances occur elsewhere. In eastern North America, there are large populations and industrial centres in the northeastern United States. Emissions of SO2 and NOx from those areas often waft into eastern Canada, worsening damage caused there by local emissions. U.S. emissions are responsible for about 90% of the wet deposition of acidifying nitrogen compounds in eastern Canada, along with 63% of the wet deposition of sulphur compounds, 43% of the dry deposition of nitrogen, and 24% of the dry deposition of sulphur (Shannon and Lecht, 1986). Canada also exports some of its emissions to the United States, although Canadian emissions account for less than 5% of the total deposition of sulphur and nitrogen compounds in the eastern states. In total, Canada receives about 4 million tonnes of SO2 per year from the United States (Environment Canada, 1999).
Dry Deposition
Dry deposition occurs during intervals between precipitation events, and it includes the following:
- the direct uptake of gaseous SO2 and NOx by vegetation, soil, and water
- gravitational settling of larger particles
- the filtering of suspended particulates by vegetation
Dry deposition occurs to all kinds of habitat, but forest is particularly effective at absorbing gases and particles from the atmosphere. This is because trees have such a large and complex surface area of foliage and bark, which greatly enhances the rate of dry deposition.
Dry deposition can result in large inputs of substances from the atmosphere, including some that generate acidity when they are chemically transformed within the ecosystem. For instance, atmospheric SO2 readily dissolves into the surface water of lakes and streams. This gas is also freely absorbed by plants – it enters foliage through tiny abundant pores on the surface known as stomata, and then dissolves into the moist film of water that covers the surface of cells in a sub-stomatal cavity. In this sense, the SO2 behaves just like CO2, a vital nutrient, which is also absorbed by plants in this manner. The absorbed SO2 becomes oxidized to the anion sulphite (SO32-), which is rapidly oxidized to sulphate (SO42-). Because the sulphate is balanced electrochemically mostly by hydrogen ions, acidity is generated by the transformation of SO2 gas into the SO42- ion (see Figure 19.2).
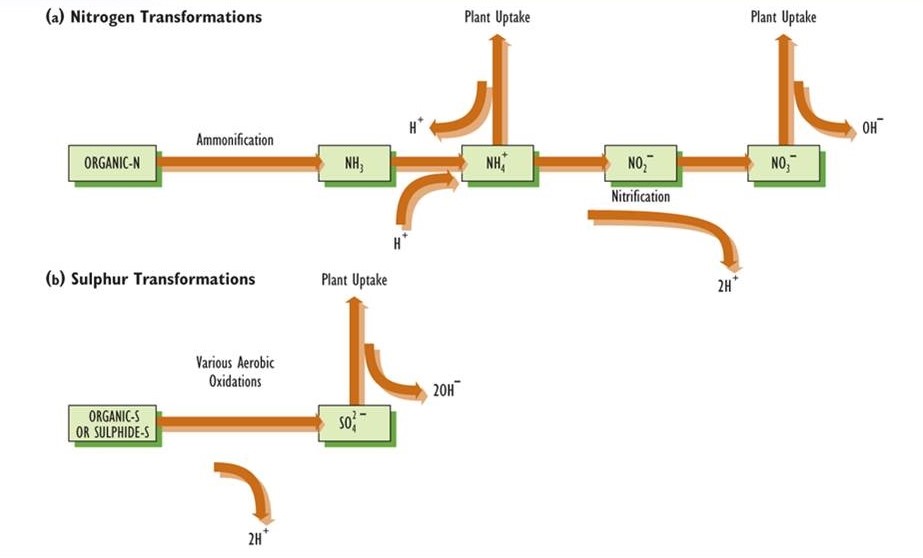
NOx gas may be similarly dry-deposited and then oxidized to nitrate (NO3-), which also generates an equivalent amount of H+. The gas ammonia (NH3) and the cation ammonium (NH4+) can also be dry-deposited to soil or water, where they can be oxidized by bacteria to nitrate plus equivalent quantities of H+.
The rates of dry deposition of sulphur and nitrogen compounds are greatest when there are high concentrations of gaseous NOx and SO2 in the atmosphere. Such conditions typically occur in urban areas and close to large industrial sources of emissions. In those places, dry deposition accounts for much larger inputs of acidifying substances than does wet deposition. In more remote, less-contaminated environments, far from sources of emission, inputs with precipitation are typically larger than dry deposition.
Within 40 km of the largest smelter at Sudbury, about 55% of the atmospheric sulphur deposition occurs as dry deposition (Chan et al., 1984). About 91% of the dry deposition involves gaseous SO2, while the rest is sulphate particulates. However, the superstack at that smelter, being extremely tall (380 m), is effective at dispersing its emissions of SO2. Consequently, less than 1% of the SO2 emitted by that point-source is deposited nearby (in this case, within 40 km). Rather, almost all of the SO2 is transported much farther away before it is deposited to the landscape.
Soil Acidity
Soil acidity is an important factor that affects the growth of plants. Soil acidification is a natural process that has been demonstrated by studies of succession in ecosystems.
One well-known study was done at Glacier Bay in Alaska. The melting of a glacier in a long fiord is exposing a mineral substrate of till that has a pH of about 8.0 and contains up to 10% carbonate minerals of calcium and magnesium (Crocker and Major, 1955). Once exposed, this material becomes modified by colonizing plants and climatic factors. Rainfall is especially important, because much of it percolates through the soil and leaches dissolved chemicals to beyond the rooting depth of the plants. These influences result in an increased acidity of the soil, which reaches about pH 4.8 after 70 years of succession, by which time a conifer forest is established. The acidification is accompanied by large and progressive declines in the amounts of Ca, Mg, and carbonates in the soil during the succession.
In part, the acidification is caused by the uptake of the nutrients Ca, Mg, and K by trees and other plants, a process that is accompanied by the excretion of H+ and a decrease in the buffering capacity of the soil (this is related to the ability of the soil to resist further acidification). The leaching of calcium and other cations out of the soil by rainwater also contributes to acidification.
Various chemical changes occur as rainwater percolates through the soil and interacts with minerals, organic matter, microbes, and roots:
- Roots and microorganisms selectively absorb, release, and transform chemicals
- Ions are exchanged at the surfaces of clay particles, minerals, and organic matter
- Insoluble minerals are made soluble by so-called weathering processes, including reactions with acids
- Secondary minerals are formed, such as certain clays and insoluble precipitates of iron and aluminum oxides
These reactions cause important changes to occur in the soil, such as acidification, the leaching of calcium and magnesium, and the solubilization of metals, particularly toxic ions of aluminum (such as Al3+; see Chapter 18). All of these processes occur naturally wherever the input of water from precipitation exceeds the amount returned to the atmosphere by evapotranspiration, so there is a surplus to percolate downward through the soil. These reactions are also influenced by the kind of vegetation growing on the site. For instance, pines, spruces, and oaks tend to cause soil to acidify. In addition, the deposition of acidifying substances from the atmosphere can potentially increase the rates of some of these processes in soil, and thereby increase the leaching of toxic Al3+ and H+ into streams and lakes.
Factors Affecting Soil Acidity
Soil acidity is influenced by numerous chemical transformations and ion exchanges. Some are carried out by organisms, while others are non-biological reactions. The following are the most important factors affecting soil acidity.
- Carbonic Acid. In many terrestrial ecosystems, such as grassland and forest, the surface litter and upper soil are rich in organic matter and roots. Decomposition and respiration result in high concentrations of CO2 (often exceeding 1%) in the atmosphere within the soil. The high CO2concentrations result in carbonic acid (H2CO3) forming in the soil water, which contributes to its acidification. This effect is strongest in soil with a pH greater than about 6.0, and it is unimportant in acidic soil with pH less than about 5.5.
- The Nitrogen Cycle. Soil acidity can also be affected by microbial transformations of nitrogen compounds and by their uptake and release by plants (Figure 19.2). Ammonium (NH4+) and nitrate (NO3-) are especially important because plants must take up one or both of these essential nutrients, the choice depending largely on soil acidity. In soil with pH less than about 5.5, almost all inorganic nitrogen occurs as NH4+. The NH4+ may originate from the ammonification of organic nitrogen to form ammonia (NH3), a process carried out by many species of microorganisms (see Chapter 5). The ammonia absorbs one H+ to form ammonium. If the NH4+ ion is absorbed by a plant root, one H+ is excreted into the soil to maintain electrochemical neutrality, so there is no net change in acidity. However, if NH4+ is added directly to soil (such as by atmospheric deposition or by the application of a fertilizer), then plant uptake of NH4+, accompanied by the release of H+, has an acidifying effect. In soils with pH greater than 5.5, most of the inorganic nitrogen occurs as NO3-, which is produced by the oxidation of NH4+ through the process of nitrification (Chapter 5). Nitrification is carried out by the bacteria Nitrosomonas and Nitrobacter, which are intolerant of acidity. The oxidation of NH4+ to NO3– generates two H+ (Figure 19.2). If the NH4+ originated from ammonification of organic nitrogen (which consumes one H+ for each NH4+ produced), the net effect is the release of one H+ for each NO3– produced from organic nitrogen. However, if the NO3– is then absorbed by a root, one OH– is excreted to the soil to maintain electrochemical neutrality, which is equivalent to the consumption of one H+. In that case, the net effect on soil acidity is zero. It is well known to farmers and agronomists that the addition of ammonium to soil can have a severely acidifying influence. This happens because the NH4+ becomes nitrified into NO3-, which generates large amounts of acidity. There are two main types of ammonium inputs: the treatment of agricultural fields with fertilizer containing inorganic nitrogen (such as urea or ammonium nitrate), and the deposition of NH3 gas and NH4+ from the atmosphere.
- The Sulphur Cycle. Much of the sulphur in soil occurs in organically bound forms. Microbial processes can transform this organic sulphur into more highly oxidized compounds, including sulphides and elemental sulphur, but if oxygen is abundant, these become further oxidized to sulphate. Overall, the oxidation of organic sulphur to SO42- releases one equivalent of H+ per equivalent of SO42- produced (this is the same as two H+ per SO42- SO42- is absorbed by a root, an equivalent quantity of OH– is excreted to conserve electrochemical neutrality, so there is no net effect on acidity. However, if atmospheric deposition causes a direct input of SO42- to the soil, followed by uptake by plant roots, the net effect is a reduction of acidity. In addition, if the soil is deficient in oxygen (anaerobic), as commonly occurs in wet sites, then the SO42- can be transformed by microbes into a sulphide compound, which results in the consumption of an equivalent amount of H+ and a reduction in acidity.
Reactions associated with the sulphur cycle usually have a smaller effect on soil acidity than those involving the nitrogen cycle. In certain situations, however, the sulphur cycle is dominant. For instance, when a wetland is drained, its previously anaerobic sediment becomes aerobic. This allows bacteria to oxidize reduced sulphide compounds into sulphate. Some drained wetlands develop an extremely acidic condition known as acid sulphate soil. For 10 or more years after drainage occurs, usually to develop agricultural land, the pH can be lower than 3.0. This severely impairs crop growth, although the acidity can be neutralized by adding calcium carbonate (lime) to the soil.
Sometimes, sulphide minerals such as pyrite (iron sulphides) become exposed to atmospheric oxygen. This allows specialized Thiobacillus bacteria to oxidize the sulphides, a process that produces sulphate and oxidized iron ions, according to the following reaction: 4 FeS2 + 15 O2 + 14 H2O → 4 Fe(OH)3 + 16 H+ + 8 SO42-
This phenomenon, known as acid-mine drainage (or as acid-rock drainage), causes severe acidification of soil and surface waters. It can cause a pH less than 2.0 to develop, with high concentrations of sulphate and toxic ions of aluminum and iron. Acid-mine drainage is an important problem where coal and metal mining have exposed mineral sulphides to the atmosphere (see In Detail 19.3).
- Uptake of Basic Cations by Plants. Terrestrial plants obtain many of their nutrients by absorbing ions from the soil in which they are growing. (A few nutrients, however, are absorbed mainly from the atmosphere, particularly CO2.) Calcium, magnesium, and potassium are important nutrients that are absorbed from soil as cations (Ca2+, Mg2+, and K+), whose absorption is offset by a release of H+. In natural ecosystems, the absorbed Ca2+, Mg2+, and K+ are eventually returned to the soil with plant litter, so there is no long-term effect on soil acidity. However, if biomass is removed from the site, as occurs in agriculture and forestry, these cations are removed, resulting in acidification of the soil.
- Leaching of Ions. In most soils, the anions nitrate and chloride readily leach downward into the groundwater. They do this because they are highly soluble in water, and are only weakly retained at anion-exchange sites on organic matter and clays. The leached anions may eventually reach surface waters such as streams and lakes. This is also true of sulphate, especially in relatively young soils of glaciated regions, including most of Canada (older soils of more southern regions often have a greater capacity to retain sulphate). In areas with large inputs of acidifying substances, the amounts of NO3– and SO42- in soil may be high enough to result in substantial rates of leaching. As these anions leach from the soil, they are accompanied by cations such as Ca2+, Mg2+, H+, and Al3+, which results in acidification, nutrient loss, and toxicity (associated with the Al3+) in terrestrial and aquatic ecosystems. For instance, one monitored watershed in south-central Ontario was found to have lost 30% of its soil calcium between 1983 and 1999 (Watmough and Dillon, 2004).
Atmospheric Deposition and Soil
The potential effects of atmospheric deposition on soil acidity have been studied in experiments in which simulated rainwater solutions was added to soil contained in plastic cylinders, known as lysimeters. These experiments have shown that extremely acidic solutions can cause these changes in soil chemistry:
- an increase in acidity
- increased leaching of calcium, magnesium, and potassium, resulting in their depletion and greater vulnerability of the soil to acidification
- increased solubilization of toxic metal ions, especially of aluminum, but also iron, manganese, and others
- an overwhelming of the ability of soil to absorb sulphate, after which this ion leaches freely, at a rate similar to its input (because SO42- is an anion, its leaching is accompanied by base cations and toxic Al3+ and H+, which may contribute to the acidification and toxicity of surface waters.)
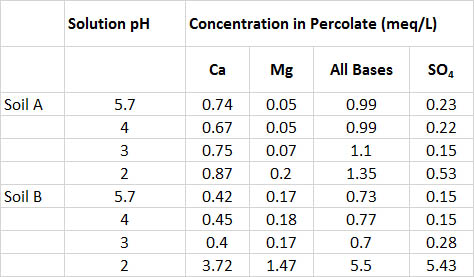
One experiment involved the treatment of a sandy soil from jack pine stands with simulated rainwater, adjusted with sulphuric acid to a pH of 5.7, 4.0, 3.0, or 2.0 (Table 19.4). Even treatment with the extremely acidic pH 2.0 had little effect on soil acidity, and the percolating solutions had a pH higher than 6.5 in all treatments. However, the leaching of Ca, Mg, total bases (Ca + Mg + K + Na), and sulphate were much higher in the pH 2.0 treatment. Overall, this experiment found that the soil was quite resistant to effects of acid loading. Eventually, however, the resistance could be overcome by treatment with highly acidic solutions, and perhaps by long-term exposure to more moderate acidities. Keep in mind that experiments such as these are short-term investigations, whereas soil acidification in nature is a slow, long-term process.
Researchers monitoring soil chemistry at particular places in the field can determine whether acidification has occurred, although such studies do not necessarily identify the causes of the change. For instance, the conversion of agricultural land into conifer forest usually results in acidification of the soil. In southern Ontario, the afforestation of abandoned farmland with pine or spruce caused the soil to acidify from pH 5.7 to pH 4.7 after 46 years of forest development (Brand et al., 1986). It is less understood whether already-forested sites will become more acidic because of atmospheric inputs of acidifying substances. A study in southern Ontario re-sampled forest soils after a 16-year interval, in a region where the average pH of precipitation is about 4.1, but no further soil acidification was observed (Linzon and Temple, 1980).
Overall, studies conducted elsewhere in Canada, and in the United States and Europe, have also come to ambiguous conclusions about the effects of atmospheric deposition on soil acidification. Except for cases where the atmosphere is severely polluted by SO2, such as near a metal smelter, there is no convincing evidence that atmospheric deposition has acidified soil on a wide scale. It appears that soil acidification is a potential long-term risk associated with this type of pollution.
Terrestrial Vegetation
Numerous studies have demonstrated that plants may be injured by treatment with simulated “acid rain.” In almost all of the studies, however, the pH that caused acute injuries was more acidic than is normally found in ambient precipitation.
For example, experiments in Norway exposed young conifer stands to simulated acid rain for three years (Tveite, 1980). The control treatment was pH 5.6-6.1, while the acidified treatments used pH 4.0 or 3.0. On average, control saplings of lodgepole pine (Pinus contorta) grew 15–20% less than plants receiving the acidic treatments. Growth of Scotch pine (P. sylvestris) and birch (Betula pendula) was also stimulated by the acidic treatments, while spruce (Picea abies) was unaffected. However, the moss-dominated ground vegetation was severely damaged by the most acidic treatment (pH 3.0).
Laboratory experiments are also useful for determining the effects of rainwater pH on plants, because the environmental conditions can be well controlled. In general, such experiments do not find reductions of growth until the pH becomes more acidic than about 3.0 (for comparison, the average acidity of precipitation is about pH 4.0 in regions where acid rain is considered a severe problem). Moreover, the productivity of some tolerant species may be stimulated by rainwater even more acidic than pH 3.0. For example, seedlings of white pine (Pinus strobus) grew more quickly when exposed to acidic mist ranging from pH 2.3 to 4.0 than at pH 5.6 (Wood and Bormann, 1976). In another experiment, seedlings of 11 tree species were treated with solutions of various pHs, but acute injury to foliage was caused only after a week of treatment at pH 2.6, which is an unnaturally acidic exposure (Percy, 1986).
In general, it appears that trees and other vascular plants have little risk of suffering acute injury from exposure to ambient acid rain. However, stresses associated with acidic precipitation could possibly decrease plant growth, even in the absence of acute injuries. These “hidden injuries” (see Chapter 16) might be caused by a subtle disruption of plant metabolism, or indirectly by changes in soil chemistry. Because acidic precipitation affects extensive regions, even a small decrease in plant productivity could have important economic and ecological consequences.
Hidden injuries, if they do occur, are most relevant to forest and other kinds of natural vegetation. Agricultural land becomes acidified mostly through management practices, such as cropping and the use of nitrogen fertilizer. Moreover, agricultural soil is routinely treated with liming agents to reduce its acidity.

A number of studies in eastern North America and Europe have examined the potential effects of acidic precipitation on forest productivity. Although species of trees in some regions have shown recent decreases in productivity, it has not been conclusively demonstrated that these changes were caused by acidic precipitation or other kinds of atmospheric pollution. Forest productivity decreases naturally as a stand matures, mainly because canopy closure intensifies competition among the trees. Forest productivity is also influenced by such factors as climate change and management practices. So far, field research has not clearly separated any influences of acidic precipitation on forest productivity from effects related to succession, climate change, insect defoliation, or other factors. However, some growth-modelling studies have suggested that productivity could decrease by 10% in eastern Canada if critical deposition rates of sulphate and nitrate are exceeded (Natural Resources Canada, 1998).
Clearly, the effects of acidifying depositions on soil and vegetation are somewhat ambiguous. However, as the following sections will show, the effects on vulnerable freshwater ecosystems can be severe.
Surface Waters
Surface waters include streams, rivers, lakes, and ponds. Their chemistry is influenced by the types of soil and vegetation in the watershed, climatic factors, and the deposition of chemicals from the atmosphere.
In regions where the winters are cold and a snowpack accumulates, the springtime meltwater that flows into streams and lakes tends to be relatively acidic. This so-called acid shock phenomenon occurs partly because snowmelt cannot percolate into the frozen soil, so its acidity does not become neutralized by interaction with minerals there. In addition, the initial meltwaters are considerably more acidic than the later fractions. The relatively intense acidity of snowmelt is responsible for much of the toxicity of affected surface waters.
The water chemistry of two lakes in a region of Nova Scotia that is subject to acidic precipitation is described in Table 19.5. These lakes are more dilute than most fresh waters, in the sense of having a low concentration of dissolved ions, and their nutrient supply is sparse, so they are unproductive (oligotrophic). Nevertheless, these lakes have higher concentrations of dissolved substances than the precipitation that falls on them –their total ions average 440 µeq/L, compared with 135 µeq/L in precipitation. The higher concentrations are due to substances that leach from terrestrial soil and eventually migrate into the lakewater. In contrast, the concentrations of ammonium and nitrate are higher in precipitation than in the lakes, suggesting that the atmospheric inputs of these nutrients are “consumed” by biological uptake within the watershed.
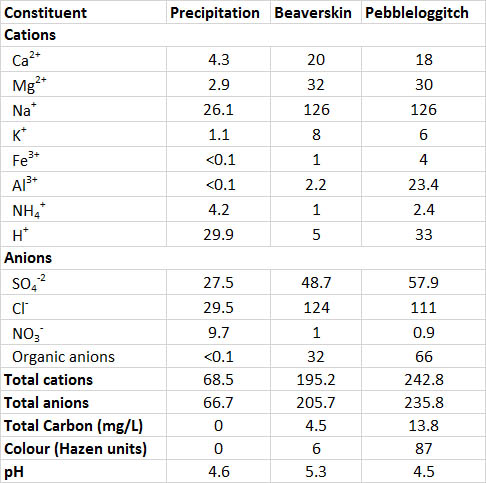
Beaverskin Lake is slightly acidic (pH 5.3) and oligotrophic, with very transparent water. In comparison, Pebbleloggitch Lake is influenced by drainage from an adjacent bog. Dissolved organic compounds (known as fulvic acids) drain from the bog into the lake, giving the lakewater a dark-brown colour and an acidity (pH 4.5) that is similar to the precipitation. Although these two lakes are located only 1 km apart, they differ markedly in acidity because of the organic acids in tea-coloured Pebbleloggitch Lake. In general, bog-influenced waters are naturally acidic, commonly with an acidity of pH 4.0-5.0.

Acidification of Surface Waters
A widespread acidification of surface waters in eastern Canada has been attributed to the deposition of acidifying substances from the atmosphere. The eastern United States and Scandinavia have also been affected in this way.
A survey of surface waters was conducted by the Environmental Protection Agency in the United States (Baker et al., 1991). In a national sample of 28,300 lakes, 1,180 were acidic, most of which are in eastern states. Atmospheric deposition was thought to have acidified 75% of the acidic lakes, while 3% were affected by acid-mine drainage and 22% by acidity from bogs. Of 64,300 streams that were sampled, 4,670 were acidic, of which 47% were acidified by atmospheric deposition, 26% by acid-mine drainage, and 27% by bogs. Florida has the highest frequency of acidic lakes, mostly because of organic acids from natural wetlands. The influence of atmospheric deposition is most important in the northeastern states, particularly in the Adirondack Mountains, where 10% of the lakes have a pH ≤5.0, and 20% have a pH ≤5.5.
Although such a comprehensive survey of the status of lakes and streams has not been carried out in Canada, acidified surface waters are known to be common, particularly in the eastern provinces. It has been estimated that there are more than 14,000 acidic lakes in Ontario, Quebec, and the Atlantic Provinces (Environment Canada, 1996). The sensitivity of surface waters to acidification is related to the amount of alkalinity in their water, which itself is associated with the amounts of calcium and magnesium in soil and rocks of the watershed (see the following section). Surface waters on 46% of Canada’s land area (about 4.0-million square kilometers) are considered highly sensitive to acidifying deposition, and another 21% (1.8-million km2) are moderately sensitive.
The chemical and biological changes that occur as surface waters become acidified have been examined in important studies in which sulphuric acid was deliberately added to lakes (Schindler, 1990). These whole-lake experiments were conducted in the Experimental Lakes Area (ELA) of northwestern Ontario. The most intensively studied lake, named Lake 223, is a 279 ha, oligotrophic waterbody. Lake 223 was studied for two years before it was experimentally acidified, and then for a number of years afterward. Beginning in 1976, sulphuric acid was added to acidify the lake, which reduced its pH from 6.5 initially to 5.0-5.1 during 1981-1983. Its acidity was then allowed to decrease to pH 5.5-5.8 during 1984-1988.
As expected, the concentrations of sulphate and hydrogen ions increased in Lake 223, because these were added to the lake. Sulphate averaged 35 µmol/L in 1975, compared with 115 µmol/L in 1979. Increased concentrations of manganese (a 980% increase by 1980), zinc (550%), and aluminum (155%) occurred because these chemicals dissolved out of sediment under the acidified conditions. Acidification also caused the water to become more transparent, which allowed increased light penetration and deeper heating during the summer. Many biological changes also occurred; these are described later in this chapter.
In Detail 19.3. Acid-rock Drainage.
Acid-rock drainage (ARD; also known as acid-mine drainage) refers to flows of water that have been severely acidified by the oxidation of pyrite and other reduced-sulphur minerals. ARD usually involves pollution associated with coal mining, but it may also be associated with metal mining. The acidity is produced by the exposure of pyritic minerals, usually iron sulphide, to atmospheric oxygen, which allows specialized Thiobacillus bacteria to oxidize the sulphides and produce sulphate and oxidized iron ions, as follows: 4 FeS2 + 15 O2 + 14 H2O → 4 Fe(OH)3 + 16 H+ + 8 SO42-
Note the large numbers of H+ that are produced – these can lead to severely acidic conditions in water draining from the substrates where the reactions are occurring. In fact, acid-mine drainage commonly results in a pH less than 2.0, along with high concentrations of sulphate and toxic aluminum, iron, and manganese. If the ARD is associated with rock or tailings from metal mining, it may also be rich in toxic copper, nickel, or other heavy metals.
As a stream flows away from a source of ARD, it interacts with neutralizing minerals and may also receive inflows of non-acidic water. These cause the acidity to be reduced, so the pH gradually increases. Once the pH recovers beyond about 3.0, dissolved iron comes out of solution as a yellow-orange precipitate of Fe(OH)3, sometimes known as “yellow-boy.” As the yellow-boy settles over the streambed, it can smother bottom-dwelling organisms.
Wherever it occurs, acid-rock drainage is a severe environmental problem, mostly because it is so toxic to aquatic life. Consequently, efforts are made to prevent ARD at the source, or to treat it once it has occurred. In Canada, work to address ARD is concentrated under the Mine Environment Neutral Drainage (MEND) program of Natural Resources Canada. It has been estimated that the total “liability” from ARD is equivalent to $2-5 billion if problems are not treated.
Where possible, it is prudent to avoid ARD by preventing the exposure of sulphide-bearing rocks to the atmosphere. Metal mines and construction industries often accomplish this by storing sulphide tailings under water. However, there are still many cases where they cause ARD and need to treat it to reduce its toxicity. The usual way to mitigate ARD is to add a liming agent, such as limestone (CaCO3) or lime (Ca(OH)2), which neutralizes the acid content. In a few cases, “artificial wetlands” have been constructed to deal with the problem: the ARD is input at one end of the wetland and exits at the other, with much of its acidic content having been consumed by microbes living in anaerobic sediment. This happens because the sulphate ions (SO42-) become chemically reduced to sulphides, such as iron sulphide (FeS2) or hydrogen sulphide (H2S). These reactions consume acidity, while also causing toxic metal ions to precipitate from solution.
Vulnerability to Acidification
Surface waters that are vulnerable to acidification have a low alkalinity, or acid-neutralizing capacity. As H+ is added to water, it is absorbed by acid-neutralizing reactions until the capacity to neutralize the acid is exceeded. After that point, there is a rapid decrease in pH, until a different buffering system comes into play (Figure 19.3). Bicarbonate (HCO3-) alkalinity is the critical buffering system within the circumneutral pH range of 6.0-8.0. When the buffering capacity of the available HCO3– is depleted, the water acidifies rapidly. Bicarbonate reacts with H+ to form H2O + CO2. Because this reaction neutralizes added H+, the pH does not change until the alkalinity is exhausted.
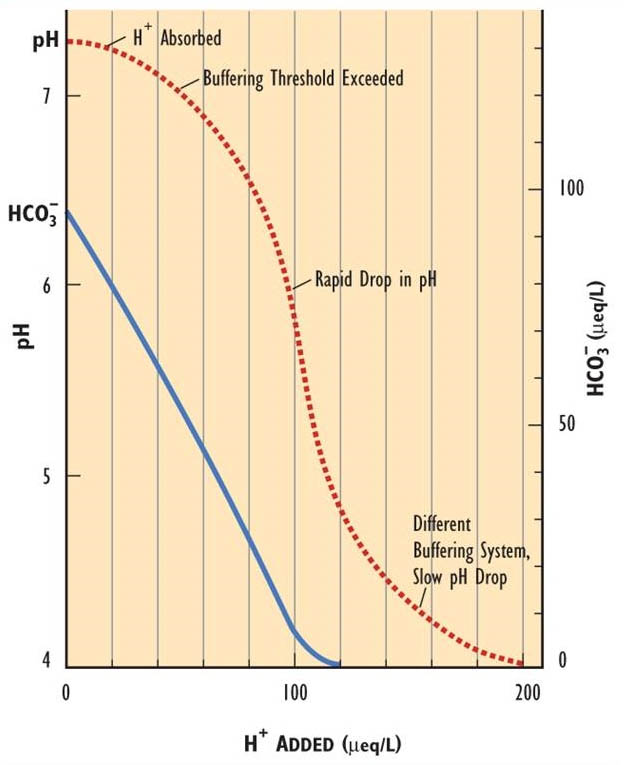
The concentration of bicarbonate in natural waters is influenced by geochemical factors, especially by the presence of limestone (CaCO3) or dolomite (Ca, MgCO3) in soil or bedrock of the watershed or in the aquatic sediment. As these minerals dissolve, they create bicarbonate alkalinity and give the water a degree of acid-neutralizing capacity. If carbonate-rich minerals are abundant in a watershed they can generate enough alkalinity to neutralize acidifying inputs from the atmosphere. As a result, surface waters in these kinds of watersheds are not sensitive to acidification, even in a region where the atmosphere and precipitation are polluted (except perhaps in cases of extremely high rates of dry deposition of SO2).
The situation is different, however, in watersheds in which the bedrock, soil, and sediment are derived from hard, poorly soluble rocks such as granite, gneiss, and quartzite, which contain few carbonate minerals. Watersheds of this type have little capacity for generating alkalinity and so they are easily acidified by wet and dry depositions from the atmosphere. Vulnerable watersheds are especially common in eastern Canada, where thin soils derived from carbonate-poor glacial till commonly overlie hard granitic bedrock.
Headwater lakes and streams are at particular risk of acidification. These systems receive no drainage from waterbodies at higher elevation, and their watersheds are usually small. Consequently, there is little opportunity for rainwater to interact with soil and bedrock, and much of the acidity in precipitation does not become neutralized before the water reaches headwater lakes and streams.
At high elevation in many mountainous regions, crustal granite is exposed by erosion and the terrain in such regions may also be vulnerable to acidification. This is the case in parts of the Rocky Mountains of western North America and in the Appalachians of the eastern United States. Again, the vulnerability occurs because granite contributes little alkalinity to surface waters.
Within a vulnerable region, however, even a small pocket of calcium-rich soil in the watershed can provide enough alkalinity to allow a waterbody to resist acidification. For example, 15 lakes were surveyed in an area of Precambrian Shield in southern Ontario where precipitation is quite acidic (Dillon et al., 1977). Fourteen of the lakes had little alkalinity (95-175 µeq/L), were slightly acidic (pH 5.8–6.7), and were considered highly vulnerable to acidification. One lake, however, had some calcium-rich till in its watershed. That lake had a high alkalinity (1,200 µeq/L) and high pH (7.1), and it is unlikely to acidify.
A related issue is the gradual loss of calcium and magnesium from terrestrial watershed due to long periods of leaching by acidic groundwater. Eventually, the supply of those cations also becomes depleted in oligotrophic streams and lakes with naturally dilute concentrations of ions, which are common in regions of granitic bedrock, such on the Precambrian Shield. The depletion of calcium and magnesium represents a depletion of the acid-neutralizing capacity of a waterbody, and it is a physiological issue for organisms that need large amounts of those cations to form their shells of calcium carbonate (such as mussels) or chitin (a glucose-derived polysaccharide that needs calcium to harden). The occurrence of aquatic osteoporosis has been demonstrated in lakes in eastern Canada, and additional research will likely show that it is widespread problem (Jeziorski et al., 2008).
Freshwater Organisms
Many changes occur in the biota as freshwater ecosystems become acidified. In general, freshwater organisms are considerably more sensitive to the acidification of their habitat than are terrestrial plants.
Freshwater Algae
Many species of microscopic, single-celled algae (phytoplankton) live in lakes. The water chemistry greatly influences the particular species that are present. Because species of diatoms (family Bacilliarophyceae) and golden-brown algae (Chrysophyceae) are particularly sensitive, they are useful indicators of water chemistry. For example, a study of 72 lakes in the Sudbury area found that certain diatom species were indicators of particular aspects of water chemistry (Dixit et al., 1991):
- indicators of acidic water: Eunotia pectinatus, Fragilaria acidobiontica, Pinnularia subcapitata, Tabellaria quadriseptata
- indicators of acidic water with high metals (Cu, Ni): Eunotia exigua, E. tenella, Frustulina rhomboides saxonica, Pinnularia hilseana
- indicators of non-acidic water: Achnanthes lewisiana, Cyclotella meneghiniana, Fragilaria construens, F. crotonensis The silica-rich cell walls of diatoms differ in shape for each species, and they persist in lake sediment after death of the cell. As well, the water-chemistry requirements of many diatom species are known. Consequently, the abundance of diatom fossils in dated layers of lake-sediment cores can be used to infer historical communities and water chemistry. This technique has been used to demonstrate that some presently acidic lakes in eastern Canada were not acidic as recently as several decades ago.
The phytoplankton community changed markedly during the acidification of Lake 223 in the Experimental Lakes Area (Findlay and Kasian, 1986). Initially, it was dominated by species of golden-brown algae, but with acidification this changed to green algae (Chlorophyceae). Although species composition changed substantially, there was little difference in the diversity of species. A small increase in algal biomass occurred as a result of the acidification, likely caused by increased water clarity, which allowed more productivity to occur. When Lake 223 was allowed to become less acidic, algal species typical of the pre-acidification community quickly reappeared.
However, the phytoplankton community is much more sensitive to fertility of the water than to changes in its acidity, being especially responsive to phosphorus (see Chapter 20). In fact, the productivity of almost all fresh waters increases if they are fertilized with phosphorus. This ecological change, known as eutrophication, also occurs in acidic waterbodies. This is illustrated by studies of two adjacent lakes in Nova Scotia, Little Springfield and Drain. After construction activity in their watersheds exposed pyrite-containing minerals to the atmosphere, both lakes became highly acidic through a process similar to acid-mine drainage (Kerekes et al., 1984). Little Springfield Lake had a pH of 3.7 and supported little algal productivity – it was oligotrophic. However, Drain Lake (pH 4.0) received phosphorus-rich sewage, and it became eutrophic and highly productive in spite of its acidity.
Periphytes are microscopic algae that live on the surface of sediment, rocks, woody debris, and aquatic plants. The periphyton community can include hundreds of species, even in acidic lakes. Periphytes are especially abundant in lakes with clear water, including acidic ones, where their late-summer biomass may develop cloudy or felt-like mats. During the acidification of Lake 223, a benthic mat of the filamentous green alga Mougeotia developed in shallow water after the pH decreased below 5.6. The reasons for the growth of algal mats are not known, but they could be due to reduced grazing by invertebrates.
Aquatic Plants
Aquatic plants (or macrophytes) can be abundant in shallow lakes and ponds. The acidification of some lakes has resulted in an increase of aquatic mosses, especially species of Sphagnum. In some cases, this was accompanied by declines of other plants, such as reed (Phragmites communis), water lobelia (Lobelia dortmanna), and quillwort (Isoetes spp.). Moreover, the invasion by Sphagnum may intensity acidification, because these mosses are highly efficient at absorbing Ca2+, Mg2+, and other cations from the water, which they exchange for H+. Mats of Sphagnum also interfere with chemical reactions at the sediment/water interface, which hinders the neutralization of acidity that occurs there.
Communities of aquatic plants differ greatly between clear-water acidic lakes and those with organically stained water. For example, Pebbleloggitch Lake (Table 19.5) has dark-brown, acidic (pH 4.5) water, which prevents light penetration into deeper habitats. Consequently, aquatic plants can grow only within a shallow fringe around the edge of the lake, and only 15% of the bottom is vegetated (Stewart and Freedman, 1989). The most abundant macrophytes, such as the yellow water-lily (Nuphar variegatum), have floating leaves. In comparison, nearby Beaverskin Lake has extremely clear water and virtually the entire bottom receives enough light to support aquatic plants, even to a depth of 6.5 m. Many of the macrophytes, including mats of Sphagnum, maintain all of their foliage underwater.
Even in acidic lakes, the productivity of macrophytes is stimulated by the addition of nutrients. Drain Lake (mentioned above) is an extremely acidic (pH 4.0) but eutrophic lake, with a lush productivity of aquatic plants. As with phytoplankton, the fertility of the water has a much greater effect on the productivity of aquatic plants than acidity does.
Zooplankton
Zooplankton are tiny animals, mostly crustaceans, that live in the water column. Most zooplankters filter-feed on phytoplankton cells, but a few are predators. Some species are tolerant of acidity and may occur in water of pH 4.0 or less. The effects of acidification on zooplankton are complex because several factors are involved:
- the toxicity of H+ and metals, such as Al3+
- changes in the availability of phytoplankton as food
- changes in predation, especially if zooplankton-eating fish are eliminated
A survey of 47 lakes in Ontario found that certain zooplankton are good indicators of water chemistry (Sprules, 1975). Indicator species of acidic lakes with pH < 5.0 were Daphnia catawba, D. pulicaria and Polyphemus pediculus. Others occurred only at pH > 5.0: Daphnia ambigua, D. galeata mendotae, D. longiremis, D. retrocurva, Diaptomus oregonensis, Epischura lacustris, Leptodora kindtii, and Tropocyclops prasinus mexicanus. However, some zooplankters were indifferent to acidity and occurred over a wide range of pH. For example, Diaptomus minutus was the most frequently observed species, occurring over pH 3.8 to 7.0. Acidic lakes had a somewhat depauperate community of zooplankters – those with pH < 5.0 had 1-7 species with only one or two dominant being dominant, while lakes with a pH > 5.0 had 9-16 species with three or four being dominant.
The experimental acidification of Lake 223 resulted in an increased abundance of zooplankton, an effect that was attributed to an increase in their food of phytoplankton biomass (Malley et al., 1982). Throughout the acidification, Diaptomus minutus and Cyclops bicuspidatus remained the most abundant zooplankters, but some other species were intolerant. This included the opossum shrimp (Mysis relicta), a large predator that disappeared when the pH decreased below 5.6.
Benthic Invertebrates
Benthic invertebrates live on or in the sediment of waterbodies. The number of species tends to be lower in acidic waters, but they can still be abundant, especially it predatory fish have disappeared. The most common benthic invertebrates in acidic lakes are species of insects and crustaceans (although other species of these groups are intolerant of acidity). Molluscs do not occur in strongly acidic conditions because it is difficult for them to maintain their shell of calcium carbonate. A study of more than a thousand lakes in Norway found that no species of clams could tolerate a pH below 6.0, and no snails below 5.2 (Okland and Okland, 1986).
Because sediment is more strongly buffered than its overlying water, it is much less vulnerable to acidification. For instance, the acidity of sediment did not change much during the experimental acidification of Lake 223 (Kelly et al., 1984). When the pH of water just above the sediment was 5.3, at 0.5 cm into the sediment it was 6.0, and at 2.0 cm it was 6.7, unchanged from the pre-acidification condition. Because the habitat of benthic invertebrates is well buffered, some of them are not much affected by acidification of the overlying lakewater. During the acidification of Lake 223, the abundance of chironomid midges increased and peaked at a water pH of about 5.6 (Mills, 1984). However, the initially abundant mayfly larvae disappeared at pH 5.0, and the crayfish Orconectes virilis became extirpated because of reproductive failure after the pH fell below 5.6.
Fish
Fish populations are the best-known victims of acidification. Many losses of trout, salmon, and other economically important fish have occurred in acidified waters in Canada, the United States, and Eurasia.
Studies in Ontario have documented the loss of fish populations from acidified lakes in the Killarney region (Beamish and Harvey, 1972; Harvey and Lee, 1982). That area is subject to severely acidic precipitation (pH 4.0-4.5) and to dry deposition of acidifying SO2 because of its proximity to the smelters at Sudbury. A survey in the 1970s found that 33 of 150 lakes in the Killarney area had a pH below 4.5. These ecologists actually monitored the local extirpation of several fishes in Lumsden and George Lakes. There was also anecdotal evidence for losses of other populations, because local people had a memory of historical sport fisheries in currently fishless lakes. The Killarney area has had 17 extirpations of lake trout (Salvelinus namaycush), an important sportfish that cannot breed at pH <5.5. There are also extirpations of smallmouth bass (Micropterus dolomieiu) from 12 lakes, of largemouth bass (M. salmoides) and walleye (Stizostedion vitreum) from four lakes, and yellow perch (Perca flavescens) and rock bass (Ambloplites rupestris) from two lakes.
Lumsden Lake acidified from pH 6.8 in 1961 to 4.4 in 1971. That acidification resulted in a reproductive failure and extirpation of lake trout, lake herring (Coregonus artedii), and white sucker (Catostomus commersoni). When George Lake reached pH 4.8-5.3, lake trout, walleye, burbot (Lota lota), and smallmouth bass disappeared. As acidification progressed further, there were losses of northern pike (Esox lucius), rock bass, pumpkinseed sunfish (Lepomis gibbosus), brown bullhead (Ictalurus nebulosus), and white sucker. These extirpations resulted from persistent failures of these fishes to reproduce in acidified lakes.
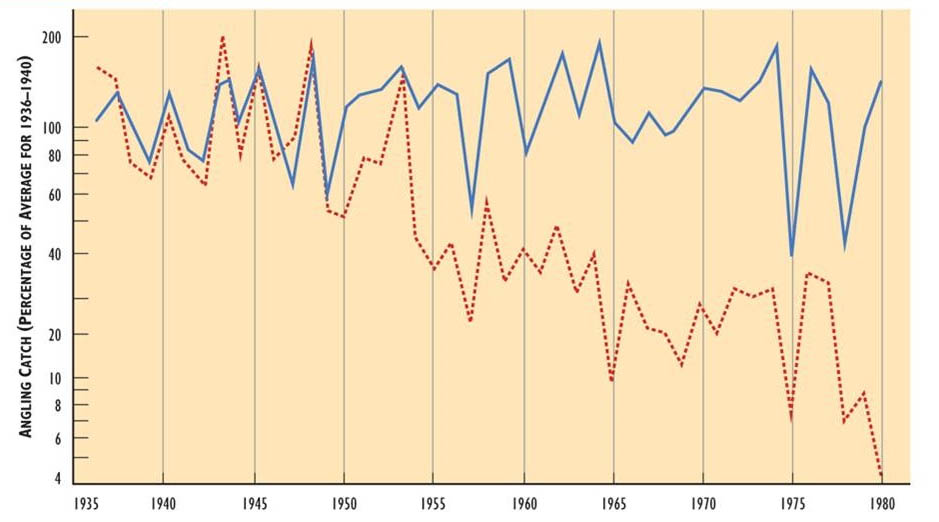
Losses of sportfish populations have also occurred in Nova Scotia, where Atlantic salmon (Salmo salar) has been lost from acidic (pH below 4.7) rivers, but not from those with higher pH (Figure 19.4). Lacroix and Townsend (1987) penned juvenile salmon in four acidic streams in Nova Scotia – none survived in pH below 4.7, but all did at higher pHs.
Elsewhere, early surveys (from the 1930s) in the Adirondack Mountains of New York State found that brook trout (Salvelinus fontinalis) were present in 82% of the lakes. However, in the 1970s, that species was absent in 43% of 215 lakes in the same region (Schofield, 1982).
An extensive survey of 700 Norwegian lakes in the 1970s found that brown trout (Salmo trutta) was absent from 40% of the waterbodies and sparse in another 40% (Wright and Snekvik, 1978). Almost all of those lakes had supported trout before the 1950s. The extirpation of trout populations was most extensive in southern Norway, where acidifying deposition is most intense.
In eastern North America, the fish that are most tolerant of acidification are yellow perch, rock bass, central mudminnow (Umbra limni), largemouth bass, bluegill (Lepomis macrochirus), black bullhead (Ictalurus melas), brown bullhead, golden shiner (Notemigonus crysoleucas), and American eel (Anguilla rostrata). These species occur in some waterbodies with pH more acidic than 4.6. Other species are more sensitive to acidification and generally need a pH >6.0 to survive.
Prior to its experimental acidification, Lake 223 supported lake trout, white sucker, fathead minnow (Pimephales promelas), pearl dace (Semotilus margarita), and slimy sculpin (Cottus cognatus) (Mills et al., 1987). The fathead minnow was most sensitive to acidification, and it rapidly declined when the pH reached 5.6. Reproductive failure of lake trout began at pH 5.4, and of white sucker at pH 5.1, resulting in population declines as older fish died.
In general, younger life-history stages (fry and juveniles) are more sensitive to acidity than are adult fishes. This is why most losses of populations are attributed to reproductive failure rather than to mortality of adults. There are, however, many observations of adult fish being killed by exposure to acid-shock events during snowmelt in the springtime.
As surface waters acidify, the concentration of dissolved metals increases, especially of Al3+ and AlOH2+, which are toxic ions of aluminum. In many acidic waters, aluminum toxicity is sufficient to kill fish, regardless of any direct effects of H+. In general, the survival and growth of fish larvae and older life-history stages become reduced when the concentration of dissolved ionic aluminum exceeds 0.1 ppm, an exposure that is common in acidic waters. In brown-coloured water, however, almost all dissolved aluminum and other metals is present as organometallic complexes. Metals in that state are much less toxic than the free-ionic forms that occur in clear water with a similar acidity.
Amphibians
Amphibians depend on aquatic habitat during at least part of their life cycle. Most Canadian species lay their eggs in water, which their larvae inhabit until metamorphosis occurs, after which the adults utilize nearby terrestrial habitat. Research suggests that some species of amphibians are vulnerable to acidification of their aquatic habitat, while others appear to be indifferent.
A study of amphibian breeding sites in Nova Scotia, covering a pH range from 3.9 to 9.0, found that some species were not obviously influenced by acidity (Dale et al., 1985):
- the bullfrog (Rana catesbeiana) occurred from pH 4.0 to 9.0
- spring peeper (Hyla crucifer) and yellow-spotted salamander (Ambystoma maculatum) from pH 3.9 to 7.8
- green frog (R. clamitans) from pH 3.9 to 7.3
- and wood frog (R. sylvatica), from pH 4.3 to 7.8.
Yellow-spotted salamander and green, bull, wood, and pickerel (R. palustris) frogs all had eggs, developing larvae, and adults present in some habitats at pH 4.0, suggesting that reproduction was occurring at that extreme acidity. Studies in other regions, however, have shown that some species of amphibians are intolerant of acidity.
Laboratory experiments with 14 species of amphibians found that exposing eggs to pH 3.7-3.9 caused more than 85% embryonic mortality, while prolonged exposure to pH 4.0 caused a rate of mortality >50% (Freda et al., 1991). It must be borne in mind, however, that an aquatic pH as acidic as 4.0 is uncommon in nature. Moreover, such acidic conditions are usually associated with acid-mine drainage or natural bogs, rather than with acidification caused by atmospheric deposition (which has an acidification threshold of about pH 4.5 or higher). Overall, it appears that most species of amphibians are less vulnerable than fish to suffering population declines caused by acidification.
Aquatic Birds
Directly toxic effects of acidification on waterfowl and other aquatic birds have not been documented and probably do not occur. However, acidification causes aquatic habitats to change, and this has indirect consequences for bird populations. For instance, if acidification eliminates populations of smaller fishes, then fish-eating waterfowl such as the common loon (Gavia immer) and merganser (Mergus merganser) will suffer, as will piscivorous raptors such as osprey (Pandion haliaetus). At the same time, however, the extirpation of predatory fish could result in an increased abundance of aquatic insects and zooplankton, which improves the food resource for other waterfowl, such as mallard (Anas platyrhynchos), black duck (A. rubripes), ring-necked duck (Aythya collaris), and goldeneye (Bucephala clangula).
The breeding success of common loons was studied on 84 lakes in Ontario (Alvo et al., 1988). Only 9% of breeding attempts were successful on low-alkalinity lakes ( < 40 µeq/L), which are either acidic or vulnerable to acidification. In contrast, 57% of breeding attempts were successful on lakes with alkalinity of 40-200 µeq/L, and 59% if > 200 µeq/L. These observations likely reflect the size of the fish populations in these lakes.
A study of 79 small lakes and ponds in New Brunswick found a larger biomass of aquatic invertebrates in the littoral zone (shallow, near-shore) in acidic waterbodies with pH 4.5-4.9 than in those with pH >5.5 (Parker et al., 1992). Five species of ducks that feed on invertebrates had an average of 3.5 broods/ha on waterbodies with pH 5.5, compared with 0.65 broods/ha at higher pHs. The greater biomass of invertebrates in the acidic waterbodies was likely due to decreased predation because of a reduced fish community.
Drain Lake in Nova Scotia was previously described as a highly acidic (pH 4.0) but eutrophic lake. Drain Lake is fishless but it has large populations of aquatic invertebrates and plants. This habitat allows black ducks and ring-necked ducks to be more productive than is typical for lakes in the region (Kerekes et al., 1984).
Reclamation
Even before acidification became a high-profile issue, wildlife managers in some regions were “improving” habitat for sportfish in brown-coloured lakes by treating the acidic water with powdered limestone (calcium carbonate, CaCO3) or lime (calcium hydroxide, Ca(OH)2). These treatments, known as liming, serve to reduce acidity, clarify the water, and improve the productivity of fish such as trout. Not surprisingly, considerable research has also been done on the use of liming treatments to improve the condition of lakes and other surface waters that have been acidified by atmospheric deposition.
Effects of liming on pH are illustrated in Figure 19.5 for three limed lakes and one reference (non-limed but acidic) lake in Ontario (Dillon et al., 1979; Yan et al., 1979). Initially, the treated lakes had a pH of 4.0-5.0, but this was increased to pH 7.0-8.0 by the whole-lake liming treatment. Middle and Hannah Lakes had a fairly stable pH after treatment, but Lohi quickly drifted back to an acidic condition. This difference reflects the sizes of the watersheds of the lakes – Lohi drains a relatively large area and flushes quickly, so its neutralization results are shorter lived. Note that fertilizing the lakes with phosphate, which stimulates the productivity of phytoplankton, also has an acid-neutralizing effect, although it is much smaller than what resulted from liming.
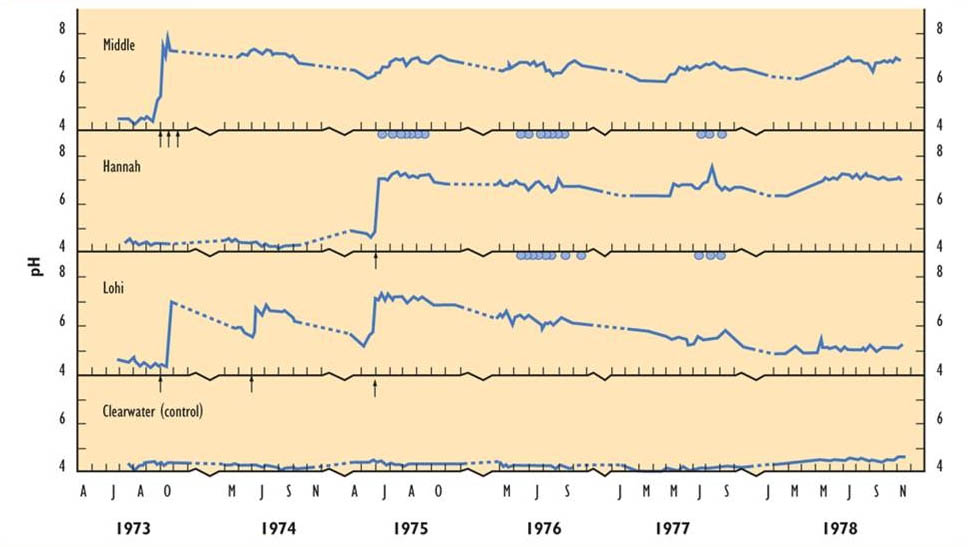
Initially, the treated lakes had a large decline in the productivity of phytoplankton and zooplankton. However, the phytoplankton biomass soon returned to the pre-liming condition, but with lingering changes in species composition. The zooplankton recovered more slowly, and even after three years had not returned to the pre-liming abundance. In addition, fish kept in cages in the limed lakes suffered high rates of mortality. This was likely due to metal toxicity, because the lakes had been affected by fallout from the Sudbury smelters. Although the aqueous concentrations of Al, Cu, Ni, and Zn all decreased after liming, because their solubility is greater in acidic water, they still remained high enough to stress fish and other biota.
In some regions of Scandinavia, liming is routinely used to treat large numbers of acidified lakes and rivers. This is done to mitigate the damage caused by acidification, especially to fish populations. Sweden, for example, has the world’s largest liming programs (Stensdotter et al., 2005). By the late 1970s, about 17-thousand lakes in Sweden had been acidified by atmospheric deposition (out of a total of 90-thousand lakes), as had many streams and rivers. Of the acidified waterbodies, more than 7,500 lakes and 14,000 km of flowing water are being treated with liming agents; about 200-thousand tonnes of powdered limestone is used each year. Typically, the liming treatment must be repeated on a three-year rotation. In Norway, about 3,000 waterbodies have been limed (State of the Environment Norway, 2008). Liming has been conducted much less extensively in North America, largely because the programs are expensive and environmental priorities are different from Scandinavia.
Research on liming has shown that acidified surface waters can be neutralized. Nevertheless, it must be understood that liming treats the symptoms of damage in acidified ecosystems, but not the causes of the acidification. Moreover, liming itself represents an environmental stress that transforms a waterbody from one polluted condition to another that is still damaged, but less toxic. Liming causes a large change in acid-adapted ecosystems, which is followed by changes in species abundances as new communities develop. In general, the most important benefit of liming is that less-acidic waters can support fish, whereas acidic waters cannot. However, liming is not a long-term solution to the acidification of fresh waters. In part, this is because waterbodies must be periodically re-treated as the liming materials are consumed or flushed out of the system.
To some degree, acidified waterbodies may also be managed by treating them with fertilizer to stimulate their productivity, as was previously examined for Drain Lake. Fertilized acidic lakes can sustain a large biomass of phytoplankton, macrophytes, and invertebrates. Waterfowl may thrive in this habitat, even if fish cannot be sustained because of the acidic conditions. However, it is not necessarily appropriate to create large numbers of highly productive lakes. For example, where recreational swimming is an important activity, abundant algae and macrophytes are considered a nuisance.

Reducing Emissions
Ultimately, the extensive damage caused by acidifying deposition from the atmosphere can only be resolved by reducing the emissions of acid-forming gases. Although this fact is intuitively clear, the issue of emissions reduction remains controversial for the following reasons:
- Scientists do not know exactly how much the emissions of SO2 and/or NOx must be reduced to prevent damage by acidifying deposition.
- Various emission-reduction strategies are possible, and they vary in their economic consequences. Would it be more effective to target large point-sources of emissions, such as power plants and smelters, while paying less attention to smaller but numerous sources, such as automobiles and oil-burning home furnaces? Or should both large and small sources be aggressively curtailed?
- To be effective, emissions reductions must be coordinated among neighbouring countries. For example, what would happen if the government of one country (perhaps with large emissions of SO2and NOx) does not regard acidifying deposition to be a high-priority problem, but neighbouring countries do?
Not surprisingly, industries and regions that are responsible for large emissions of acid-forming gases have tended to resist the imposition of substantial legislated reductions of their releases. In general, they argue that the scientific justification for the reductions is not yet convincing, while the costs of controls are known to be large and potentially disruptive of the economy.
In addition, how low should the rates of atmospheric deposition of sulphur and nitrogen compounds be, in order to avoid further acidification of sensitive surface waters or to allow their recovery? The critical rates of deposition of acidifying compounds are influenced, in part, by the vulnerability of the receiving ecosystems – areas with shallow, nutrient-poor soil can sustain much lower inputs of acidifying substances than areas rich in calcium. Highly sensitive lakes in eastern Canada are considered to have critical-load thresholds of <20 meq/ha•y of sulphur and nitrogen deposition (Figure 19.6).
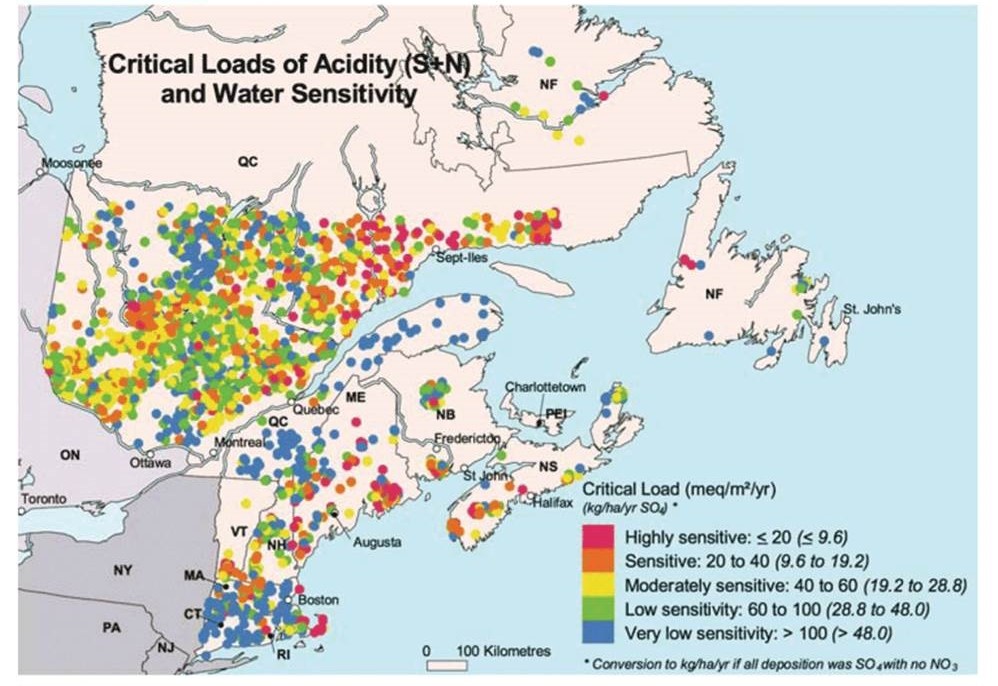
Although there are many uncertainties about the specific causes and magnitude of the damage caused by the atmospheric deposition of acidifying substances, it is obvious that what goes up (emissions of acid-precursor gases) must eventually come down (as acidifying deposition). This common sense idea is supported by a great deal of scientific evidence. This knowledge, combined with public awareness and concern about acidification in many countries, has spurred politicians to begin to take effective action. This is resulting in reduced emissions of SO2 and NOx, particularly in relatively wealthy countries in North America and Western Europe.
In 1992, the governments of Canada and the United States signed a binational treaty aimed at reducing acidifying deposition in both countries. This agreement, known as the Canada–U.S. Air Quality Agreement, calls for large expenditures by industries and governments to substantially reduce the emissions of air pollutants, especially SO2. These cutbacks are on top of reductions of emissions that both countries had already achieved during the 1980s. In the United States, emissions of SO2 have decreased from 23.1 Mt in 1980 to 16.9 Mt in 1995 and to 6.0 Mt in 2011, while emissions of NOxincreased slightly from 20.7 Mt in 1980 to 21.8 Mt in 1995 and then decreased to 13.6 Mt in 2011 (EPA, 2014; see also Table 16.2). For comparison, the Canadian emissions of SO2 decreased from 4.7 Mt in 1980 to 2.5 Mt in 1995 and to 1.3 Mt in 2012, while emissions of NOx remained about the same during 1980 to 1995 at 2.4–2.6 Mt/y, and then declined to 1.9 Mt/y in 2012 (Environment Canada, 2014).

A major component of the U.S. initiatives to reduce emissions of SO2, established under the 1990 Clean Air Act Amendments, is the creation of a marketplace for emissions trading. In essence, any company that is emitting SO2 at a rate less than it has been permitted by the Environmental Protection Agency (EPA) has a right to sell (or trade) those non-used “credits” to another business that is exceeding its emissions target. This is an important initiative because it helps to set a “market value” for emissions of certain pollutants, and also for investments to reduce their release. From the environmental perspective, this marketplace for emissions is a logical instrument because the atmosphere is a common-property resource that is owned and affected by everyone, so any changes in the release of pollutants (whether increases or decreases) have a global effect. In essence, a company whose emissions are smaller than its allowance can realize a profit by selling its credits, while another that has exceeded its target incurs costs. Those costs may be paid either by purchasing emissions credits or by taking action to reduce the emissions, such as installing SO2-removal technology, switching to a low-sulphur fuel, or in extreme cases, shutting down particularly dirty facilities.
The flexibility associated with these options is considered by many economists and politicians to have been an important benefit of the system of emissions trading. Nevertheless, the establishment of a marketplace that commodifies the release of SO2 does liberate many companies from the expensive investments that would be required to achieve tangible reductions of their emissions. This fact has engendered controversy, as has the potential establishment of a global marketplace for tradable emissions of greenhouse gases under the Kyoto Protocol (Chapter 17).
In any event, are the cuts in emissions large enough to achieve their intended effect of preventing and repairing the acidification of ecosystems? According to a science assessment carried out by Environment Canada, the reductions of SO2 emissions have resulted in lower rates of acidifying deposition in Canada (based on a comparison of data for 1990–1994 and 1996–2000; Environment Canada, 2005). Nevertheless, an estimated 21-75% of eastern Canada still receives amounts of acidifying deposition that exceed the critical loads (the smaller numbers correspond to a best-case scenario, and the larger to a worst-case one). Environment Canada also suggests that, to protect ecosystems from further damage caused by acidifying deposition, a further 75% reduction of SO2 emissions will be required by Canada and the United States beyond those agreed to under the existing Air Quality Agreement. A similar conclusion was reached by the U.S. Environmental Protection Agency in its own science assessment of the issue (2011).
Furthermore, not much action has been taken to reduce the emissions of NOx, and this appears to be working against the environmental benefits associated with increased control of SO2. It is crucial that future regulatory actions include reduced emissions of both SO2 and NOx and that acid rain and its environmental damage continues to be monitored.
The 1992 air-pollution treaty between Canada and the United States is a helpful accomplishment. Although there have been reductions in the acidity of precipitation and surface waters in some areas, it appears that the reductions of SO2 emissions are not large enough to fully mitigate many of the damages caused by acidifying deposition. So far, extremely large areas of terrain continue to be affected by acidification caused by Atmospheric deposition.
Improving trends in the chemistry of precipitation and streamwater at Hubbard Brook, NH, a monitoring location with outstanding longer-term data of this sort of data, are shown in Figure 19.7. The precipitation data show that the acidity of precipitation is decreasing (the pH is increasing), and that the concentrations of sulphate and nitrate are also decreasing. The reduction of sulphate concentrations is especially large, and likely reflects the fact that regulatory controls have concentrated on SO2 emissions (the main precursor of sulphate) more so that on NOx (the precusors of nitrate). The streamwater data also show decreasing acidity and sulphate concentration, as well as a steady decrease in the concentrations of calcium. The latter observation may reflect a progressive loss of calcium from these watersheds, which represents a degradation of the ability of the system to provide acid-neutralizing capability agains future inputs of acidifying substances. John Smol (2008) of Queen’s University refers to the phenomenon of progressive calcium losses as a kind of “osteoporosis” that affects and degrades many watersheds whose soil and bedrock are low in calcium.
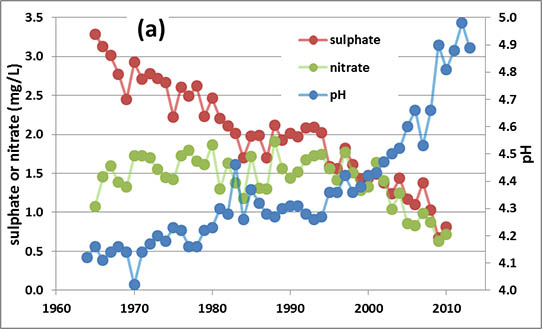
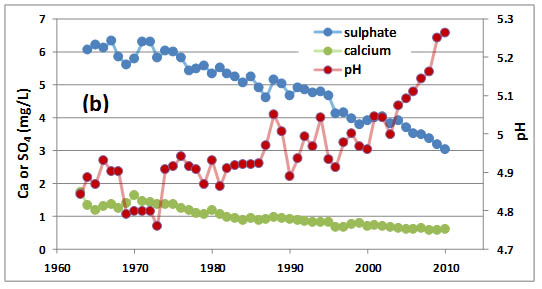
Some lakes are also benefiting from reduced sulphate loading. A survey of 202 lakes monitored in eastern Canada since the early 1980s found that the acidity of 33% had decreased, while 56% were unchanged, and 11% became more acidic (Environment Canada, 1998. Overall, about 80% of recently sampled lakes in Nova Scotia had a pH <6.0, as did 40% of those in Ontario and 25% in New Brunswick (note that pH <6.0 is considered a critical threshold of tolerance for sensitive fish and other aquatic animals; Environment Canada, 2005). Many of these lakes are naturally acidic because of organic substances leaching from bogs, particularly in Nova Scotia, where brown-waters comprise about 40% of the acidic lakes. Across eastern Canada, however, 0.5-0.6 million lakes are thought to still be vulnerable to acidification under an atmospheric-deposition regime similar to that in recent years.
It must be recognized that pollution control is extremely expensive. For example, it could cost $600 million annually to further reduce the SO2 emissions by 50% below the current targets for eastern Canada and the eastern United States. Because of this cost, policies that favour reduced emissions of SO2 and NOxmay not be able to survive the frequent challenges mounted by politicians, economists, and business people who do not believe that such actions are necessary.
So far, actions to reduce the emissions of SO2 and NOx have been vigorous only in relatively wealthy regions of North America and Western Europe. In less-wealthy countries, the political focus is mostly on industrial and economic growth. However, air pollution and other environmental damages “subsidize” that economic growth and are often paid little heed. As soon as possible, much more political and scientific attention must be devoted to the problems of acidifying deposition and other kinds of pollution in eastern Europe, Russia, China, India, Southeast Asia, Mexico, and other rapidly growing economies. In those countries, emissions of SO2, NOx, and other important airborne pollutants are galloping out of control.
Conclusions
Acidification is a natural process that occurs as ecosystems interact with climatic and biological influences, for example in bogs and coniferous forest. Acidification is also caused by anthropogenic influences, particularly emissions of SO2 and NOx, which oxidize to form acids while in the atmosphere or after they are dry-deposited to ecosystems. Aquatic and terrestrial ecosystems that are vulnerable to acidification have little acid-neutralizing capacity, largely because of small amounts of calcium and magnesium carbonates in their soil, sediment, or rocks. Low-alkalinity freshwaters are particularly at risk of acidification by atmospheric deposition. When a waterbody acidifies to a pH less than about 6.0, it begins to lose its populations of vulnerable fish species and other sensitive biota. Acidifying influences also damage the built environment by eroding materials made of limestone, marble, and certain metals such as copper. Although some of the ecological damage caused by acidification to surface waters can be mitigated by liming, this treatment has to be repeated, typically on about a three-year rotation. The best way to avoid the environmental problems associated with acidifying deposition is to reduce the emissions of the key acid-precursor gases (SO2 and NOx). To a substantial degree, this is being done in wealthy countries, including Canada. However, rapidly growing economies, such as China and India, are not paying much attention to this environmental problem, and it is rapidly becoming worse as they aggressively increase their supply of commercial energy by burning sulphurous fossil fuels, particularly coal.
Questions for Review
- Explain the principle of conservation of electrochemical neutrality? How is it relevant to the chemistry of precipitation and surface waters?
- What environmental influences cause soil to become acidic? How can this problem be mitigated?
- How does the acidification of freshwater habitat affect the aquatic biota, including phytoplankton, macrophytes, zooplankton, benthic invertebrates, fish, and birds?
- Define critical load, and explain factors that influence its value for particular kinds of terrain and surface waters.
Questions for Discussion
- Compare the chemistry of rainwater and lakewater in a region that is vulnerable to acidification. What are the reasons for the differences?
- How do wet and dry depositions of acidifying substances contribute to acidification? Why do their rates and relative importance differ between urban and rural areas?
- Are surface waters in the area where you live acidic or likely to become so? Explain your answer in terms of terrain factors that influence vulnerability to acidification.
- Explain why wealthy countries have been taking action to reduce their emissions of acid-precursor gases, but rapidly growing economies such as China and India have not. What are likely consequences of these policies?
Exploring Issues
- There are two broad options for dealing with acidifying deposition: (1) reduced emissions to prevent the problem and (2) liming of water and soil to treat the symptoms. Some people have called emissions reductions the “billion dollar solution,” and liming the “million dollar solution.” This is because of the potentially greater capital costs associated with technologies for reducing SO2 and NOx emissions. Which of these options (or both) do you think is most appropriate for dealing with acidification as an environmental problem? Explain your answer.
- Canada and the United States have negotiated a treaty concerning the transboundary movements of air pollutants, with a focus on acidifying deposition from the atmosphere. Major aspects of the treaty are reductions in the emissions of SO2, and to a lesser degree, NOx to the atmosphere. You are a scientist working for Environment Canada and have been given the responsibility of monitoring whether the negotiated emissions reductions have been successful in improving conditions in the province where you live – is acidification becoming less of a problem? How would you design a program of environmental monitoring and research to answer this important question? What ecological and human health questions would you examine?
References Cited and Further Reading
Alvo, R., D.J.J. Hussell, and M. Berrill. 1988. The breeding success of common loons (Gavia immer) in relation to alkalinity and other lake characteristics in Ontario. Canadian Journal of Zoology, 66: 746-752.
Baker, L.A., A.T. Herlihy, P.R., Kaufmann, and J.M. Eilers. 1991. Acidic lakes and streams in the United States: The role of acidic deposition. Science, 252: 1151-1154. Beamish, R.J. and H.H. Harvey. 1972. Acidification of the La Cloche Mountain Lakes, Ontario, and resulting fish mortalities. Journal of the Fisheries Research Board of Canada, 29: 1131-1143.
Brand, D.G., P. Kehoe, and M. Connors. 1986. Coniferous afforestation leads to soil acidification in central Ontario. Canadian Journal of Forest Research, 16: 1289-1391.
Brodin, Y.W. and J.C.I. Kuylenstierna. 1992. Acidification and critical loads in Nordic countries: A background. Ambio, 21: 332-338.
Buso, D.C., G.E. Likens, and J.S. Eaton. 2000. Chemistry of Precipitation, Streamwater, and Lakewater in the Hubbard Brook Ecosystem Study. General Technical Report NE-275, U.S. Department of Agriculture, Northeast Forest Experiment Station, Newtown Square, PA.
Chan, W.S., R.J. Vet, C.U. Ro, A.J.S. Tang, and M.A. Lusis. 1984. Long-term precipitation quality and wet deposition in the Sudbury basin. Atmospheric Environment, 18: 1175-1188.
Crocker, R.L. and J. Major. 1955. Soil development in relation to vegetation and surface age at Glacier Bay, Alaska. Journal of Ecology, 43: 427-448.
Dale, J.M., B. Freedman, and J. Kerekes. 1985. Acidity and associated water chemistry of amphibian habitats in Nova Scotia. Canadian Journal of Zoology, 63: 97-105.
Dillon, P.J., D.S. Jefferies, W. Snyder, R. Reid, N.D. Yan, D. Evans, J. Moss, and W.A. Scheider. 1977. Acid Rain in South-Central Ontario. Ontario Ministry of the Environment, Rexdale, ON.
Dillon, P.J., N.D. Yan, W.A. Schieder, and N. Conroy. 1979. Acidic lakes in Ontario, Canada: Characterization, extent, and responses to base and nutrient additions. Archives of Hydrobiology, Suppl. 13: 317-336.
Dixit, S.S., A.S. Dixit, and J.P. Smol. 1991. Multivariate environmental inferences based on diatom assemblages from Sudbury (Canada) lakes. Freshwater Biology, 26: 251-266.
Environment Canada. 1996. The State of Canada’s Environment. Government of Canada, Ottawa, ON.
Environment Canada. 1998. Canadian Acid Rain Assessment. Volume 1. Summary of Results. Environment Canada, Ottawa, ON.
Environment Canada. 2005. Canadian Acid Deposition Science Assessment 2004. Meteorological Service of Canada, Downsview, ON. http://publications.gc.ca/pub?id=9.688243&sl=0
Environment Canada. 2014. National Pollutant Release Inventory and Air Pollutant Emission Summaries and Trends. Environment Canada, Ottawa, ON. http://www.ec.gc.ca/inrp-npri/default.asp?lang=en&n=0EC58C98-1#Emission_Summaries Accessed January 2015.
Environmental Protection Agency (EPA). 2011. National Acid Precipitation Assessment Program Report to Congress 2011: An Integrated Assessment. US EPA, Research Triangle Park, NC. Accessed January, 2014.
Environmental Protection Agency (EPA). 2014. Air Trends. US EPA, Research Triangle Park, NC. https://www.epa.gov/air-trends Accessed January, 2014.
Findlay, D.L. and S.E.M. Kasian. 1986. Phytoplankton community responses to acidification of Lake 223, Experimental Lakes Area, northwestern Ontario. Water, Air, and Soil Pollution, 30: 719-726.
Freda, J., W.J. Sadinski, and W.A. Dunson. 1991. Long term monitoring of amphibians populations with respect to the effects of acidic deposition. Water, Air, and Soil Pollution, 55: 445-462. Freedman, B. 1995. Environmental Ecology. 2nd ed. Academic Press, San Diego, CA.
Freedman, B. and T.A. Clair. 1987. Ion mass balances and seasonal fluxes from four acidic brownwater streams in Nova Scotia. Canadian Journal of Fisheries and Aquatic Science, 44: 538-548.
Gerhardsson, L., A. Oskarsson, and S. Skerfving. 1994. Acid precipitation – effects on trace elements and human health. Science of the Total Environment, 153: 237-245.
Godbold, D.L. and A. Huttermann (eds.). 1994. Effects of Acid Rain on Forest Processes. Wiley-Liss, Hoboken, NJ.
Harvey, H.H. and C. Lee. 1982. Historical fisheries changes related to surface water pH changes in Canada. Pp. 45-55 in: Proceedings of the International Symposium on Acid Rain and Fisheries.
Henriksen, A. 1980. Acidification of freshwaters – a large scale titration. Pp. 68-74 in: Ecological Impacts of Acid Precipitation, (D. Drablos and A. Tollan, eds.). SNSF Project, Oslo, Norway.
Jeffries, D.S., T.G. Brydges, P.J. Dillon, and W. Keller. 2003. Monitoring the results of Canada/U.S.A. acid rain control programs; some lake responses. Environmental Monitoring and Assessment, 88: 3-19.
Joskow, P.L., A.D. Ellerman, J.-P. Montero, R. Schmatensee, and E.M. Bailey. 2000. Markets for Clean Air: The U.S. Acid Rain Program. Cambridge University Press, Cambridge, UK.
Kelly, C.A., J.W.M. Rudd, A. Furutani, and D.W. Schindler. 1984. Effects of lake acidification on rates of organic matter decomposition in sediments. Limnology and Oceanography, 29: 687-694.
Kerekes, J. and B. Freedman. 1988. Physical, chemical, and biological characteristics of three watersheds in Kejimkujik National Park, Nova Scotia. Archives of Environmental Contamination and Toxicology, 18: 183-200.
Kerekes, J., B. Freedman, G. Howell, and P. Clifford. 1984. Comparison of the characteristics of an acidic eutrophic and an acidic oligotrophic lake near Halifax, Nova Scotia. Water Pollution Research Journal of Canada, 19: 1-10.
Lacroix, G.L. and D.R. Townsend. 1987. Responses of juvenile Atlantic salmon (Salmo salar) to episodic increases in acidity of Nova Scotia rivers. Canadian Journal of Fisheries and Aquatic Science, 44: 1475-1484.
Linzon, S.N., and P.J. Temple. 1980. Soil resampling and pH measurements after an 18-year period in Ontario. Pp. 176-177 in: Ecological Impact of Acid Precipitation, (D. Drablos and A. Tollan, eds.). SNSF Project, Oslo, Norway.
Lovett, G.M., W.A. Reiners, and R.K. Olson. 1982. Cloud droplet deposition in subalpine balsam fir forests: Hydrological and chemical budgets. Science, 218: 1303-1304.
Malley, D.F., D.L. Findlay, and P.S.S. Chang. 1982. Ecological effects of acid precipitation on zooplankton. Pp. 297-327 in: Acid Precipitation Effects on Ecological Systems (F.M. D’Itri, ed.), Ann Arbor Science, Ann Arbor, MI.
Mills, K.H. 1984. Fish population responses to experimental acidification of a small Ontario lake. Pp. 117-131 in: Early Biotic Responses to Advancing Lake Acidification (G.R. Hendry, ed.). Butterworth, Toronto.
Mills, K.H., S.M. Chalanchuk, L.C. Mohr, and I.J. Davies. 1987. Responses of fish populations in Lake 223 to 8 years of experimental acidification. Canadian Journal of Fisheries and Aquatic Science, 44, Suppl. 1, 114-125.
Morrison, I.K. 1983. Composition of percolate from reconstructed profiles of jack pine forest soils as influenced by acid input. Pp. 195-206 in: Effects of Accumulations of Air Pollutants in Forest Ecosystems. (B. Ulrich and J. Pankrath, eds.). Reidel, Berlin, Germany.
Natural Resources Canada. 1998. Effects of Acidic Deposition on Canada’s Forests. Natural Resources Canada, Ottawa, ON.
Okland, J. and K.A. Okland. 1986. The effects of acid deposition on benthic animals in lakes and streams. Experimentia, 42: 471-486.
Parker, G.R., M.J. Petrie, and D.T. Sears. 1992. Waterfowl distribution relative to wetland acidity. Journal of Wildlife Management, 56: 268-274.
Percy, K.E. 1986. The effects of simulated acid rain on germinative capacity, growth, and morphology of forest tree seedlings. New Phytologist, 104: 473-484.
Reuss, J.R. and D.W. Johnson. 1985. Effect of soil processes on the acidification of water by acid precipitation. Journal of Environmental Quality, 14: 26-31.
Ro, C.U. 2014. Analyzed data fields from the National Atmospheric Chemistry Database (NAtChem) and Analysis Facility. Air Quality Research Division, Environment Canada, Toronto, ON. www.msc–smc.ec.gc.ca/natchem (Provided as a personal communication.)
Rose, J. (ed.). 1994. Acid Rain: Current Situation and Remedies. Gordon & Breach Science Publishers, Yverdon, Switzerland.
Scheider, W.A., D.S. Jeffries, and P.J. Dillon. 1980. Bulk deposition in the Sudbury and Muskoka–Haliburton areas of Ontario during the shutdown of INCO Ltd. in Sudbury. Ontario Ministry of the Environment, Rexdale, ON.
Schindler, D.W. 1988. Effects of acid rain on freshwater ecosystems. Science, 239: 149-157. Schindler, D.W. 1990. Experimental perturbations of whole lakes as tests of hypotheses concerning ecosystem structure and function. Oikos, 57: 25-41.
Schindler, D.W., K.H. Mills, D.F. Malley, D.L. Findlay, J.A. Shearer, I.J. Davies, M.A. Turner, G.A. Linsey, and D.A. Cruikshank. 1985. Long-term ecosystem stress: the effects of years of experimental acidification on a small lake. Science, 228: 1395-1401.
Schofield, C.L. 1982. Historical fisheries changes in the United States related to decreases in surface water pH. Pp. 57-67 in: Proceedings of the International Symposium on Acid. Rain and Fisheries. American Fisheries Society, Bethesda, MD.
Shannon, J.D. and B.M. Lecht. 1986. Estimation of source-receptor matrices for deposition of NOx–N. Water, Air, and Soil Pollution, 30: 815-824.
Sprules, W.G. 1975. Midsummer crustacean zooplankton communities in acid-stressed lakes. Journal of the Fisheries Research Board of Canada, 32: 389-395.
Stensdotter, U., T. Svenson, U. Larsson, and G. Persson. 2005. Mitigation of acidification in lakes by liming. Integrated Studies of the Effects of Liming Acidified Waters, Swedish Environmental Protection Agency, Stockholm, Sweden.
Stewart, C.C. and B. Freedman. 1989. Comparison of the macrophyte communities of a clearwater and a brown-water oligotrophic lake in Kejimkujik National Park, Nova Scotia. Water, Air, and Soil Pollution, 46: 335-341.
Tveite, B. 1980. Effects of acid precipitation on soil and forest. 9. Tree growth in field experiments. Pp. 206-207 in: Ecological Impact of Acid Precipitation (D. Drablos and A. Tollan, eds.). SNSF Project, Oslo, Norway.
Watmough, S.A. and P.J. Dillon. 2004. Major element fluxes from a coniferous watershed in central Ontario, 1982-1999. Biogeochemistry, 67: 369-398.
Watt, W.D., C.D. Scott, and W.J. White. 1983. Evidence of acidification of some Nova Scotia rivers and its impact on Atlantic salmon. Canadian Journal of Fisheries and Aquatic Science, 40: 462-473.
Wood, T. and F.H. Bormann. 1976. Short-term effects of a simulated acid rain upon the growth and nutrient relations of Pinus strobus. Pp. 815–824 in: General Technical Report NE-23, Northeastern Forest Experiment Station, U.S.D.A. Forest Service, Upper Darby, PA.
Wright, R.F. and E. Snekvik. 1978. Acid precipitation: Chemistry and fish populations in 700 lakes in southernmost Norway. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie, 20: 765-775.
Yan, N.D., R.E. Girard, and C.J. Lafrance. 1979. Survival of Rainbow Trout, Salmo gairdneri, in Submerged Enclosures in Lakes Treated with Neutralizing Agents near Sudbury, Ontario. Ontario Ministry of the Environment, Rexdale, ON.


