23: Environmental Effects of Forestry
- Page ID
- 42048
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
After completing this chapter, you will be able to:
- Explain how forest harvesting removes nutrient capital from the site.
- Outline how forestry can damage aquatic ecosystems, and how many of those effects can be avoided.
- Describe how clear-cutting affects biodiversity.
- Explain the special qualities of old-growth forest, and how they are affected by timber harvesting.
- Discuss ecological consequences of the conversion of natural forest into plantations.
- Explain the concept of integrated forest management.
Introduction
Forestry includes both the harvesting of trees and the management of post-harvest succession to foster the regeneration of another forest. Forest science guides these activities by providing an understanding of the environmental factors that affect the productivity of trees. The primary goal of commercial forestry is to provide sustainable harvests of tree biomass that can be used to manufacture lumber, paper, and other industrial products, or as a source of bio-energy. Secondary goals are related to the management of hunted animals (such as deer and fish) on forest landscapes, and sometimes the designation of certain areas as protected (where no timber harvesting occurs).
Forestry is an important economic sector in many countries. This is the case in Canada, where a large industrial enterprise depends on a continuous supply of tree biomass for the production of products that have a great economic value ($34 billion in 2013; see Chapter 14 for forestry-related economics).
Of course, to achieve these economic benefits, trees must be harvested from extensive areas of mature forest. In Canada, clear-cutting is the most commonly used method of forest harvesting – this method removes all of the economically useful trees on a site at the same time. Clear-cutting accounts for about 90% of the annual timber harvest.
However, clear-cutting is not the same as deforestation. Deforestation involves the permanent conversion of a forest into some other kind of ecosystem, such as an agricultural or urbanized land-use. In Canada and most other developed countries, industrial clear-cutting is generally followed by the regeneration of another forest. In fact, it is common practice to manage the post-harvest succession to speed up the rate of regeneration of trees. This allows the next harvest to be made after a relatively short time, so more profit can be made. (This period of time is known as a harvest rotation.) In this sense, forestry as it is usually practised does not result in a net deforestation, and if appropriately managed the forest resource is not depleted. Even though hundreds of thousands of hectares are harvested each year in Canada (638-thousand ha in 2013), the net deforestation is essentially zero (Table 14.11).
Timber harvesting can be viewed as a disturbance of the forest ecosystem, followed by regeneration. Additional disturbances are associated with silvicultural activities, such as preparing the site for planting, thinning dense stands, and applying herbicide or insecticide to deal with pest problems. Silviculture, the branch of forestry that involves tending and growing forests, is practised over an extensive area in Canada. For instance, about 67% of the area harvested (in 2012) is planted to tree seedlings (the other 33% regenerates naturally.) Some planted areas are managed intensively to develop a plantation, which is an anthropogenic forest, but one that lacks many of the ecological and aesthetic values of natural forest.
In this chapter we examine some of the ecological effects of timber harvesting and silviculture, with a focus on site quality, hunted animals, and biodiversity. Additional effects of forestry are examined in other chapters: pesticide spraying in Chapter 22, implications for carbon storage in Chapter 17, and tropical deforestation and global biodiversity in Chapter 26.
Forest Harvesting and Site Capability
In Chapter 14, we defined site capability (or site quality) as the potential of land to sustain the productivity of agricultural crops. This is also relevant to forestry, in terms of the ability of land to sustain the productivity of trees. Site capability is a complex attribute that involves the amounts of nutrients and organic matter in soil, the availability of moisture, and other factors affecting plant growth. These factors are influenced by soil type, climate, drainage, rate of nutrient cycling, and the kinds of plant and microbial communities that are present.
The ability of soil to supply plants with nutrients is a critical aspect of site capability. In large part, this ecological function depends on the nutrient capital of a site, which is the amount of nutrients present in the soil, living vegetation, and dead organic matter. When trees are harvested, the nutrients in their biomass are also removed, which can deplete the nutrient capital of the site.
A stand of forest may be harvested using a variety of methods, which vary in the amount of biomass and nutrients that are removed from the site. A selection harvest is a relatively “soft” method because it involves the harvesting of only some of the trees from a stand, leaving others behind and the structure of the forest substantially intact. The most intensive harvest is a clear-cut, in which all economically useful trees are removed. The smallest clear-cuts, typically involving a hectare or less, are known as a group-selection harvest. More typically, clear-cuts entail the harvesting of trees from larger areas, on the order of 20-100 ha. The largest clear-cuts may extend over hundreds, and even thousands, of hectares. However, such extensive operations are unusual, and are usually associated with the salvaging of trees that have been damaged by a wildfire, windstorm, or insect infestation.
There are also some less intensive methods of clear-cutting. A shelterwood harvest is a staged clear-cut, in which some larger trees of economically desirable species are left standing during the initial cut. These provide a seed source and a partially shaded environment that encourages natural regeneration. Once the regeneration is well underway, the large “leave” trees are harvested. A strip-cut is another kind of staged harvest, in which long and narrow clear-cuts are made at intervals, with uncut forest left in between to provide a source of seed to regenerate trees in the cut strips. Once the regeneration is established, another strip-cut is made, again leaving intact forest on one of the sides. This system of progressive strip-cutting continues until all the forest in the management block (the specific area being managed this way) has been harvested. Typically, an area is harvested in three to four strips. To regenerate trees on the final strips, foresters may rely on advanced regeneration – that is, on small individuals of tree species that existed in the stand prior to harvesting and that survived the disturbance of clear-cutting. Alternatively, they may plant the last strip with seedlings.

Clear-cutting systems also vary in how intensively the biomass of individual trees is harvested. The usual stem-only harvest involves the removal of tree trunks, leaving the roots, stumps, and logging “slash” (cut branches and foliage) on the site. The harvested logs can then be processed into lumber or pulp for manufacturing paper. A whole-tree harvest is more intensive because it removes all of the above-ground biomass of the trees, including the branches and foliage. This intensive kind of harvest will recover considerably more biomass than a stem-only cut, which is an advantage if the wood is to be used as a source of bio-energy.
Nutrient Losses during Harvesting
Although intensive harvests such as a whole-tree clear-cut increases the yield of biomass, there is also considerably more removal of nutrients. Some forest scientists have suggested that the nutrient removals from whole-tree harvests could degrade the capability of sites to sustain tree productivity. The problem would be especially severe if the harvests are conducted over a short rotation. This might not allow enough time for the nutrient capital to recover by natural inputs, such as by precipitation, nitrogen fixation, and weathering of minerals (Figure 23.1).
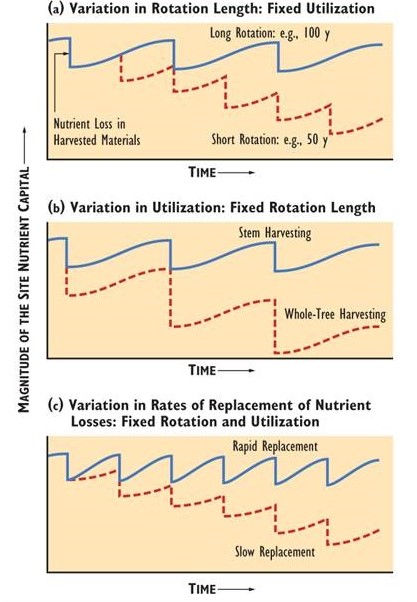
Site impoverishment caused by intensive cropping is a well-known problem in farming, in which severely degraded land may have to be abandoned for some or all agricultural purposes. However, usually this problem can be managed, to a degree, by applying fertilizer or composted organic matter to the land. Sometimes, however, the degradation of site capability, especially of tilth, is too severe, and this simple mitigation is not successful. Of course, the harvest rotation in agriculture is usually annual, whereas in forestry it ranges from about 20 to 100 years. However, each timber harvest involves the removal of a huge quantity of biomass, and thus of nutrients.
Compare, for example, the amounts of biomass and nutrients removed by clear-cuts of a conifer forest in Nova Scotia (Table 23.1). In this case, a whole-tree clear-cut yielded 30% more biomass than a stem-only harvest. The increased yield may be an advantage, particularly if the harvest is to be used for energy production. The increased harvest of biomass is, however, due to the removal of nutrient-rich tissues such as foliage and small branches. Consequently, the whole-tree harvest removed up to twice as many nutrients as did the stem-only clear-cut. In effect, a 30% increase in biomass yield by the whole-tree method was “purchased” at the ecological “expense” of 54-99% increases in the removal of nutrients.

Unfortunately, there are few studies that allow foresters to compare the productivity of subsequent harvest rotations on the same site. Such studies would take more than 50-100 years, requiring several generations of foresters! Therefore, it is difficult to evaluate the implications of nutrient removal by clear-cutting. Overall, however, it appears that a degradation of site nutrient capital is a less severe problem in forest harvesting than in agriculture. Consequently, nutrient removals by timber harvesting should be viewed as a potential long-term problem. Because forestry is an economically important activity, and the maintenance of site capability is critical to the sustainability of the enterprise, scientists should continue to study the effects of harvesting on nutrient capital. In the short term, however, forestry causes more immediate kinds of damage to site capability and biodiversity that deserve our attention.
Leaching of Nutrients
The disturbance of forested land can increase the rate at which dissolved nutrients are transported downward into the soil with percolating rainwater (a process known as leaching). If the nutrients leach deeper into the soil than tree roots can penetrate, they are effectively lost from the “working” nutrient capital of the site. Eventually, leached nutrients can find their way into groundwater and surface waters.
The nutrients with the greatest tendency to leach are nitrate and potassium, both of which are highly soluble in water. However, calcium, magnesium, and sulphate may also leach in significant amounts. Of course, following a clear-cut, any nutrient losses by leaching are in addition to that removed with tree biomass.
A well-known study of nutrient leaching caused by forest disturbance was done at Hubbard Brook, New Hampshire. This large-scale experiment involved felling all of the trees on a 16-ha watershed, but without removing any biomass – the cut trees were left on the ground. The watershed was then treated with herbicide for three years to suppress regeneration. This experiment was designed to examine the effects of intense disturbance, by de-vegetation, on biological control of watershed functions such as nutrient cycling and hydrology. The research was not intended to examine the effects of a typical forestry practice.
Overall, during a 10-year period following the cutting, the de-vegetated watershed lost 50 kg/ha-year of NO3-N (i.e., nitrogen in the form of nitrate), 45 kg/ha-y of Ca, and 17 kg/ha-y of K in streamflow (Bormann and Likens, 1994; Figure 23.2 shows data for the first three years, which were the most dramatic). The losses were much larger than from an undisturbed reference watershed: 4.3 kg/ha-y of NO3-N, 13 kg/ha-y of Ca, and 2.2 kg/ha-y of K.
In part, the increased losses of nutrients were due to a 31% increase in the yield of water from the de-vegetated watershed (during the first three years after cutting). The increased streamflow was caused by the disruption of transpiration from plant foliage. However, increases in nutrient concentration in the streamwater were more important: during the first three years, NO3 increased by an average factor of 40; K, by 11; Ca, by 5.2; and Mg, by 3.9. The losses of N, Ca, and Mg in the streamwater were similar to their amounts in the above-ground biomass of the forest.
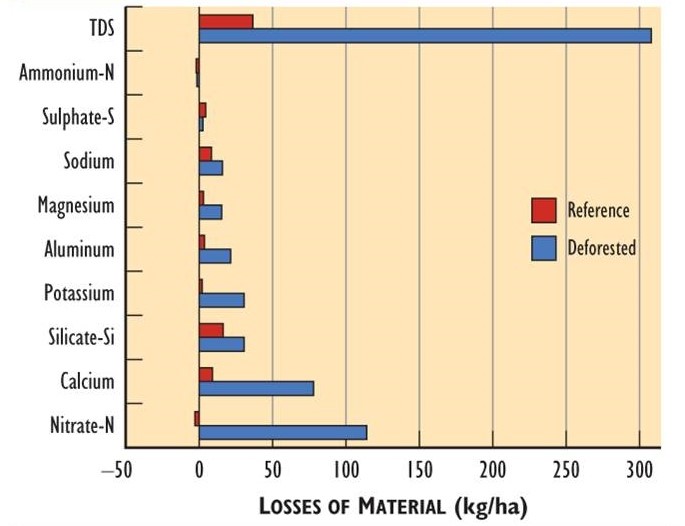
Because this experiment in de-vegetation did not involve a typical forestry practice, the measured effects are unrealistically large. However, watershed-level studies of clear-cutting have also found an increase in nutrient leaching, although to a lesser degree than that caused by the de-vegetation at Hubbard Brook. For example, in the first three years after clear-cutting a 391 ha watershed in New Brunswick, there was an increased loss of nitrate in streamwater of 7 kg NO3-N/ha-yr (Krause, 1982). A study of nine clear-cut watersheds in New Hampshire found an average nitrate loss of 18 kg NO3-N/ha-y during the first four years, compared with 3.5 kg/ha-y for five uncut watersheds (Martin et al., 1986). In addition, calcium losses from the clear-cuts averaged 28 kg/ha-y, compared with 13 kg/ha-y for reference watersheds, while potassium losses were 6 kg/ha-y compared with 2 kg/ha-y. However, other studies have found smaller effects of clear-cutting on nutrient losses with streamflow, especially if only a portion of the watershed was cut.
Nitrate and other highly soluble ions are leached from watersheds after clear-cutting (and after other disturbances, such as wildfire) for several reasons. First, disturbance stimulates the activity of microbes involved in the decomposition of organic matter. This occurs because removal of the tree canopy results in warming of the forest floor and surface soil, and decreased uptake by plants leads to an increase of soil nutrients and moisture. Second, disturbance often stimulates the microbial processes of ammonification and nitrification (see Chapter 5), resulting in increased rates of production of nitrate, which is extremely soluble and readily lost from soil.
Forestry and Erosion
Forestry activities can cause severe losses of soil, or erosion, particularly in terrain with steep slopes. In most cases, erosion is triggered by improperly constructing logging roads, using streams as trails to haul logs, running log-removal trails down slopes instead of along them, and harvesting trees from steep slopes that are extremely vulnerable to soil loss. In general, however, road building is the most important cause of erosion on forestry lands, especially where culverts (channelled stream crossings) are not sufficiently large or numerous, or are poorly installed.
Severe erosion causes many environmental damages. In extreme cases, the loss of soil may expose bedrock, making forest regeneration impossible. Soil loss also represents a depletion of site nutrient capital. Erosion also causes secondary damage to aquatic habitats, including the deposition of silt (or siltation), which covers gravel substrates that are important to spawning fish. Also, the shallower water increases the risk of flooding.
However, in many cases erosion is a largely avoidable environmental effect of forestry. The irresponsible practices that can cause erosion are restricted by provincial regulations and occur much less frequently now than in the past. Practices that help to reduce erosion include the following:
- planning the route of forest roads to avoid stream crossings as much as possible
- installing a sufficient number of properly sized culverts
- avoiding the disturbance of stream channels by heavy equipment
- leaving buffer strips of uncut forest beside watercourses
- using log-removal practices that avoid disturbance of the forest floor (such as cable yarding, in which a tall spar anchors cables radiating into the clear-cut, which allows logs to be dragged to a central place without the use of a wheeled skidder)
- allowing vegetation to regenerate quickly, which speeds the re-establishment of biological moderation of erosion
- deciding to selectively harvest, or to not harvest, steep sites that are highly vulnerable to erosion
It has become a common practice to leave strips of uncut forest beside streams, rivers, and lakes. These buffer zones greatly reduce the erosion of streambanks, eliminate temperature increases in the water, maintain riparian (lake- and stream-side) habitat for wildlife, and mitigate some of the aesthetic damage from forest harvesting.
While it is widely accepted that riparian buffers provide important benefits, there is no consensus about how wide the uncut strips should be. This is an economically important consideration, because large areas of valuable timber are withdrawn from the potential harvest when buffer strips are left. The requirements in New Brunswick, for example, are for a 30-m buffer on each side of a watercourse, with wider buffers recommended in some circumstances (such as 60 m if the slope exceeds 24°, and up to 100 m beside waters that are used for recreation or as a source of drinking water).
In some cases, selective harvesting of trees may be allowed within riparian buffers, as long as this does not compromise the ecological services provided by these special management zones.
Forestry and Hydrology
The cover of forest on a watershed has a strong influence on its hydrology. Large amounts of water are evaporated into the atmosphere by vegetation, especially by trees because they have so much foliage (this is transpiration; evapotranspiration includes evaporation from non-living surfaces). In the absence of transpiration, an equivalent quantity of water would leave the watershed as streamflow or as seepage to deeper groundwater.
For example, studies of four well-forested watersheds in Nova Scotia found that evapotranspiration was equivalent to 15-29% of the annual input of water by precipitation (rain plus snow), with runoff in streams accounting for the other 71-85% (these watersheds have no substantial drainage to deep groundwater; Freedman et al., 1985).
The hydrologic budget of watersheds is extremely seasonal, particularly in the temperate and boreal climates that are typical of forested regions of Canada. This can be illustrated by the watershed of the Mersey River in Nova Scotia (Figure 23.3). The annual input of water from precipitation was 146 cm/y, with 82% arriving as rain and 18% as snow. About 62% of the annual input was dispersed by riverflow and 38% by evapotranspiration. Evapotranspiration is highest during the growing season, which results in relatively sparse riverflow. Runoff is greater during late autumn and early winter, when there is little transpiration because deciduous trees have dropped their foliage, and even conifers are in a quiescent state. However, much of the precipitation during that period recharges groundwater storage, which had been depleted by the uptake of water by vegetation during the summer. Runoff is greatest during the spring, when the accumulated snowpack melts, and that results in a spate of riverflow.

Disturbances such as wildfire and timber harvesting alter the hydrology of watersheds. The seasonality and amounts of flow can change, and erosion and flooding may occur downstream. Some poorly drained sites may become wetter, because reduced transpiration can raise the water table. In general, the increase in streamflow is related to the proportion of the watershed that was disturbed. If an entire watershed is clear-cut, the increase in streamflow can be as much as 40% in the first year. The increase is proportionately less after partial cuts.
Clear-cuts usually regenerate quickly, and in some cases the vigorous regrowth of shrubs and herbs can restore most of the original foliage area in as few as four to six years. Consequently, the biggest increases in streamflow occur in the first year after cutting, followed by rapid recovery to the pre-harvest condition. In the temperate and boreal climates prevalent in much of Canada, the largest increases in streamflow occur during the late spring, summer, and early autumn, these being the seasons when transpiration is most important.
Hydrology can also be affected by a change in the type of forest that is dominant on a watershed. For example, if an area of hardwood forest is converted into conifer plantations, the annual streamflow may decrease. This happens because the conifers maintain their foliage throughout the year, and so extend the transpiration season into times when angiosperm trees lack foliage.
Weeds and Reorganization
Clear-cuts usually regenerate rather quickly. Initially, however, most of the regenerating biomass involves plants other than the tree species that foresters consider desirable. As a result, the vigorous regrowth is often regarded as being detrimental to silvicultural objectives. Such non-crop plants may be viewed as “weeds,” and their abundance may be controlled by a herbicide application (see Chapter 22).
However, a rapid re-vegetation of clear-cuts and other disturbed lands does provide important ecological benefits. The regenerating plants influence the ecological “reorganization” of disturbed lands. They re-establish a measure of biological control over nutrient cycling, erosion, and hydrology, while also restoring habitat for animals.
For example, during the first few years after clear-cutting, fast-growing vegetation restores a high rate of nutrient uptake from the soil. Through this uptake, the regenerating vegetation acts as a “sponge” that absorbs some of the soluble nutrients that might otherwise leach from the site. Eventually, the early successional plants die, and their nutrients are recycled by decomposition and made available to trees. In addition, the re-vegetation restores habitat for birds, mammals, and other wildlife. Clearly, the early reorganization phase of succession is enhanced by the rapid regeneration of many plant species, including those considered to be weeds by foresters.
Forestry and Biodiversity
Clear-cutting and other forestry practices inflict intense disturbances on forests. They cause dramatic changes in the habitat available to support plants, animals, and microbes, as well as their various communities. Some species benefit from habitat changes that occur because of forestry, but others suffer damage.
In the following sections, we examine the effects of forestry on aspects of Canadian biodiversity – the richness of biological variation in our country. The effects of clear-cutting on plants, mammals, birds, and fish will be examined because these groups are relatively well studied and are considered to be important by our society.
Vegetation
Any severe disturbance results in changes in the dominant species of plants that are living on a site. Because they have such a great influence on local environmental conditions, trees are the dominant organisms in forests. After clear-cutting, many smaller plants take advantage of the relatively uncompetitive conditions that occur, and they dominate the initial stages of the post-harvest succession. They are then reduced in abundance, or even eliminated from the community, once several decades of regeneration have gone by and tree-sized plants re-establish their dominance.
Many plants of early post-cutting succession can only be successful in open habitats –they are intolerant of the shade and other stressful conditions beneath a forest canopy. These relatively short-lived ruderal plants can typically disperse widely, a propagation strategy made necessary because of their ephemeral habitat (see Chapter 9). Ruderal plants that can proliferate in recently disturbed forests include asters, goldenrods, grasses, and sedges. A specific example is the fireweed (Chamerion angustifolium), a purple-flowered herb that is often abundant after wildfire (hence its name) and also after clear-cutting. Some woody plants are also ruderals, because they are most abundant during the recovery after disturbance. Examples are the red raspberry (Rubus strigosus), pin cherry (Prunus pensylvanica), and elderberry (Sambucus racemosa). Because of their need for open, recently disrupted habitat, ruderal plants benefit from clear-cutting and other disturbances that are associated with forestry.
Unlike ruderal plants, some other species are tolerant of the environmental stress that occurs beneath a closed forest canopy. Examples are the white trillium (Trillium grandiflorum), shield fern (Dryopteris marginalis), feather mosses (such as Pleurozium schreberi and Hylocomium splendens), and certain lichens, such as lungwort (Lobaria pulmonaria). These species are not tolerant of open conditions, and they decline after clear-cutting. Once suitable conditions re-develop, these plants may again increase in abundance.
In general, once a clear-cut has had two to four years to regenerate, the plant community is actually richer in species than the mature forest that was harvested (this is particularly true of vascular plants). The increase in species diversity occurs because recently disturbed habitats are relatively flush in such resources as light, nutrients, and water. This allows many species of low-growing plants to be supported, including a diversity of ruderal ones. In comparison, stressful habitats, such as the understorey beneath a mature forest canopy, support fewer species of plants.
A study of a hardwood forest in Nova Scotia illustrates the species-rich nature of vegetation after a clear-cut (Crowell and Freedman, 1994). That study examined stands of mature forest, plus clear-cuts of various age. The number of plant species in the ground vegetation (shorter than 2 m) averaged 11/m2 on two one-year-old clear-cuts, and increased to 14/m2 on six-year-old clear-cuts. Mature forest and clear-cuts older than 30 years had fewer species – only 3-6/m2 in stands with a closed canopy dominated by maple trees, but 9-11/m2 in birch-dominated stands, which have a more open canopy. This comparison suggests that many plants, especially ruderals, can utilize open habitats associated with clear-cutting. However, species that need mature forest as habitat are threatened by this kind of disturbance.
Deer, Moose, Elk, and Caribou
White-tailed deer (Odocoileus virginianus) and mule deer (O. hemionus) are the most common wild ungulates in southern Canada. They feed on woody stems (known as browse) and low-growing herbaceous plants, and they need brushy habitat for at least part of their yearly range. The abundance of these deer has increased in many regions since the European colonization of Canada, prior to which landscapes were more extensively covered with mature and old-growth forest. In Nova Scotia, for example, white-tailed deer were initially uncommon and were soon extirpated by over-hunting. However, these deer were re-established in the nineteenth century by deliberate introductions and natural immigration from New Brunswick. Today, this species is likely more abundant in the region than at any time since deglaciation.
The modern abundance of Odocoileus deer is largely due to an increased availability of early successional, shrubby habitat, along with decreased populations of their natural predators. The shrubby habitat was created by the abandonment of poorer-quality agricultural land, timber harvesting, and wildfire. These all result in habitat dominated for several decades by shrub-sized plants, with a rich understorey of forbs (herbaceous dicot plants) and graminoids (grasses, sedges, and rushes).
The shrubby habitat tends to be distributed on the landscape as a mosaic of stands in various stages of succession within a matrix of mature forest. This spatial arrangement enhances the suitability of the landscape for Odocoileus if the following conditions are present: (1) extensive production of nutritious and palatable browse in younger stands; (2) abundance of ecotonal (or edge) habitat; (3) availability of good yarding habitat of mature conifer forest, which provides shelter in regions where the winter is severe and the snowpack is deep. If these habitat qualities occur within a mosaic of stands of various ages, the landscape is more favourable to these deer than either extensive clear-cuts or unbroken expanses of mature forest.
The central parts of a large clear-cut are not well used by deer, as they like to be close to protective forest cover. A study in eastern Canada found that white-tailed deer fed about seven times more intensively in the centre of clear-cuts less than 80 ha in area than in the middle of larger clear-cuts up to 410 hectares in size (Drolet, 1978). In fact, optimal clear-cuts for white-tailed deer are rather small in area, although this varies regionally. For example, clear-cuts smaller than 4 hectares in New Brunswick and smaller than 2 hectares in southern Ontario have been recommended to improve habitat for white-tailed deer.
To some degree, the amount of useful habitat on larger clear-cuts is greater if they have an irregular shape. Erratic shapes have a higher ratio of edge to area than do circular, square, or rectangular shapes. Consequently, an irregular shaped clear-cut provides more edge habitat, while also making the central part of the harvested area more accessible to deer.
Clear-cuts may also have physical obstructions to deer movements, such as tangles of logging slash. They may also have deep snow during the winter because snowfall is not intercepted by an overhead canopy of conifer trees. In general, deer movements are severely restricted by snow deeper than 50-70 cm.
Deer eat a variety of woody plants, forbs, and graminoids, and these foods are often much more abundant on cutover and burned sites than in mature forest. After clear-cutting, the biomass of browse and herbaceous plants typically increases to a peak after 8-15 years, followed by a decline as the tree canopy matures and shades the understorey. This successional pattern is illustrated in Figure 23.4 for stands of various ages following clear-cutting. The quantity of browse peaked at 8-13 years and then declined. The pattern for herbaceous plants was similar, but the biomass peaked at two to six years.
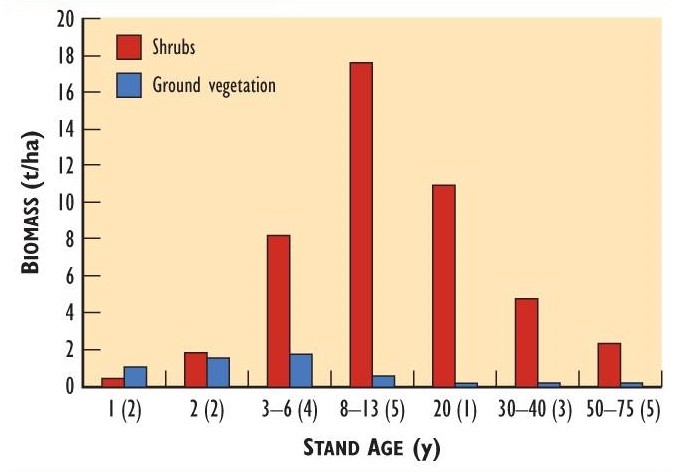
Browse is not only more abundant, but also of better nutritional quality in clear-cuts and burns than in mature forest. Recently sprouted, rapidly growing twigs have higher concentrations of protein, nitrogen, and phosphorus, and are more succulent and more easily digested than the older browse that is found in mature forest.
Ongoing disturbances in forest management areas can also affect habitat use by deer. These include frequent traffic along logging roads, noise from harvesting operations, and the excessive hunting pressure that can result from easy access along forestry roads.
Moose (Alces alces) and elk (Cervus elaphus) may also benefit from some forest-harvesting practices. Moose feed primarily on browse, although they also eat aquatic and terrestrial forbs during the summer. Elk graze on graminoids and forbs during the growing season, but eat browse during the winter. Because the abundance of browse and herbs usually increases after timber harvesting, the habitat of moose and elk can be somewhat improved. In general, however, these species are less favoured by forestry than are white-tailed and mule deer.
The woodland caribou (Rangifer tarandus; known as reindeer in Eurasia) is another abundant species of deer, particularly in the north. They require an extensive habitat of mature conifer forest, particularly during the winter when “reindeer mosses” (actually species of Cladina lichens) make up much of their diet. These lichens grow on the forest floor and are most abundant in open conifer stands that are 40 to 100 years old. However, if the tree density is high enough to allow the canopy to close, these lichens decline and are replaced by feather mosses, which are not palatable to caribou. The disturbance of forests by wildfire and logging can regenerate the supply of reindeer lichens in areas with extensive closed-canopied stands. In general, however, caribou are not favoured by extensive logging of their habitat.
All of the species of wild ungulates in Canada are important in hunting, an activity that generates economic value while also providing subsistence for many rural people (see Chapter 14). Increasingly, foresters and wildlife biologists are working together to develop integrated management plans that can accommodate the need to harvest both timber and ungulates from landscapes. These plans can allow relatively large populations of white-tailed deer, mule deer, moose, and elk to occur, even while clear-cutting and other forestry practices take place. In general, however, caribou do not do well in regions where a great deal of timber harvesting occurs, and they are also intolerant of other industrial activities, such as those related to oil and gas. We will examine integrated forest management in more detail at the end of this chapter.
Smaller Mammals
Hares and rabbits are abundant in most regions of Canada and are economically important as small game, as pests, and in recreational wildlife viewing. They feed by browsing and grazing and can benefit from an increase of low shrubs and herbs after timber harvesting, the abandonment of agricultural land, and wildfire. In fact, hares and rabbits can be abundant enough to impede tree regeneration through girdling (gnawing the bark around a sapling, which kills the young tree) and clipping (chewing the foliage and growing points of a young tree).
Other small mammals, such as mice, voles, shrews, and moles, are important as components of food webs. They also sometimes impede forest regeneration by consuming tree seeds and by girdling saplings. In some cases, they are considered beneficial because they eat potentially damaging insects.
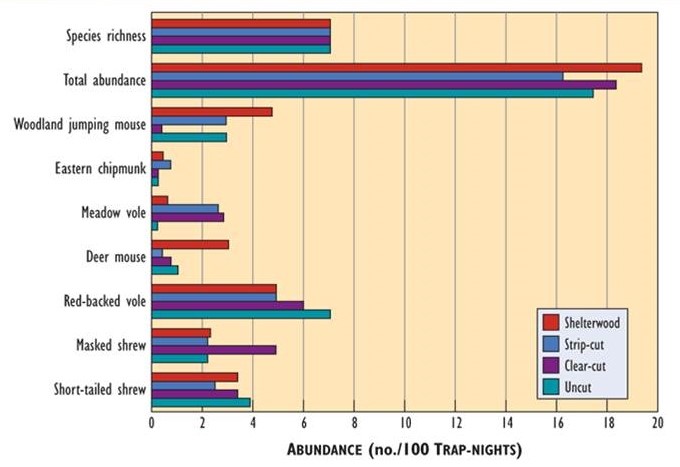
Most studies report that forest harvesting has relatively minor effects on small mammals. For example, no substantial differences were found in their overall abundance, species richness, or diversity among stands of mature forest, three- to five-year-old clear-cuts, strip-cuts, and shelterwood cuts (Figure 23.5).
Pine marten (Martes americana) and fisher (M. pennanti) are medium-sized carnivores with extensive ranges in North America. Unfortunately, these furbearers have suffered large population declines in many regions, mostly because they have been trapped too intensively. In addition, marten and fisher appear to depend partly on the complex habitat structure of older coniferous forest. Consequently, they are considered to be at risk from timber harvesting.
Birds
Many birds require mature forest as habitat for breeding, wintering, or during migration. However, many other species need the types of habitat that occur during early stages of forest succession, including those created through such forestry activities as clear-cutting.
Ruffed grouse (Bonasa umbellus) are commonly hunted, as are spruce grouse (Canachites canadensis) and blue grouse (Dendragapus obscurus). Populations of these “upland game birds” are generally favoured by a landscape mosaic that includes both mature forest and younger, brushy stands. Ruffed grouse prefer areas dominated by hardwood forest with some conifers mixed in, especially stands dominated by poplars and birches. These birds feed mainly on the foliage, young twigs, catkins, and buds of woody plants, and also eat fleshy fruits when available. In Nova Scotia, they utilize clear-cuts of maple-birch forest that are five or more years old. Clear-cuts of aspen forest in Minnesota become suitable after 4 to 12 years of regeneration, and are then used for 10 to 15 years, while older aspen stands are wintering habitat (Gullion, 1988). Wildlife biologists recommend that, to provide habitat for ruffed grouse in aspen forest, forestry should be conducted to create a mosaic of different-aged stands, each of 10 hectares or less, with adjacent ones differing in age by 10 to 15 years.
Wildlife managers sometimes refer to the many birds that are not hunted as “non-game” species. These birds can, however, be economically important as predators of insects that damage trees or other crops, and as the object of bird-watching (or birding), a popular outdoor sport. Forestry affects these birds and their communities by changing the physical structure and plant-species composition of the available habitat.
An important aspect of habitat structure is the distribution of distinct patches, either within a stand or on a landscape. The shape of a patch affects its ratio of edge to area, and thus the amount of ecotonal habitat. Patch size is also important because small, isolated habitats cannot sustain birds that maintain a large territory. In addition, the species composition of the vegetation affects the types of food and other habitat elements that occur in a stand. The presence of cavity trees, standing dead trees (or snags), and logs on the forest floor is also critical to many birds and other animals (this is discussed in the next section). Finally, the abundance of many birds often increases in stands in which there is an outbreak of insects, such as spruce budworm (see Chapter 22).
Many birders have a general knowledge of the relationships between bird species and habitats, and they use this understanding to predict what they might see under certain conditions. Ecologists know enough about the specific requirements of some birds to manage their habitat. The best forestry-related example of this practice is the use of prescribed fire to create even-aged stands of jack pine (Pinus banksiana) in Michigan. This ensures an appropriate habitat for the endangered Kirtland’s warbler (Dendroica kirtlandii).
Of course, each bird species has particular habitat needs. If the physical and botanical character of a habitat is changed by a disturbance such as wildfire or clear-cutting, many species can no longer breed in the affected stand. The same disturbance, however, will create opportunities for early successional birds.
These changes are illustrated in Table 23.2, which compares the birds in mature stands and in clear-cuts of hardwood forest. The mature forest supported an average population of 663 pairs/km2, dominated by ovenbird, least flycatcher, red-eyed vireo, black-throated green warbler, and hermit thrush. The three- to five-year-old clear-cuts supported a slightly less abundant population of 588 pairs/km2, dominated by chestnut-sided warbler, common yellowthroat, white-throated sparrow, and dark-eyed junco. Note that although the forest and clear-cuts had similar densities of birds, the species were almost entirely different. This occurred because the habitats were very different in terms of physical structure and the species composition and biomass of vegetation. Although clear-cutting deprived mature-forest birds of habitat, it created opportunities for early successional species.
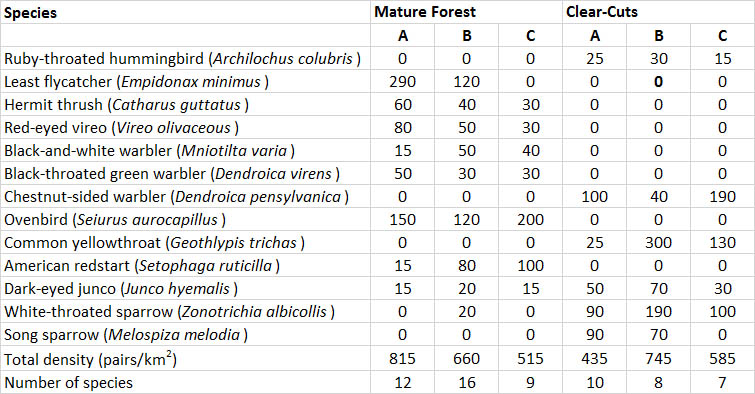
Welsh and Fillman (1980) examined the effects on birds of clear-cutting spruce forest in northern Ontario. The largest populations of birds (1020-1970 pairs/km2) occurred in moderate-aged (11 to 24 years) clear-cuts. This was a higher abundance than occurred in uncut forest (561 pairs/km2). The lowest densities of birds occurred in a three-year-old clear-cut (200 pairs/km2). By five years after the cutting, this had increased to 690 pairs/km2. In general, the clear-cuts and mature forest supported different birds, although there was some overlap.

Image 23.3. This is an eight-year-old clear-cut of hardwood forest. Birds breeding in this habitat include chestnut-sided warbler (Dendroica pensylvanica), common yellowthroat (Geothlypis trichas), and alder flycatcher (Empidonax alnorum). Source: B. Freedman. 
Cavity Trees and Deadwood
Living trees with heart-rot cavities, standing dead trees (snags), and logs lying on the forest floor (coarse or large woody debris) are critical habitat elements for many animals. This is particularly true of many birds, which use these habitat features for nesting, as substrates for foraging, and as perches for hunting, resting, or singing. In fact, about one-third of the birds that breed in temperate and boreal forest depend on these features, particularly on cavities.
For example, all 12 species of woodpeckers that breed in Canada excavate cavities in snags or in living, heart-rotted trees. The cavities are used as nesting sites and for roosting at night. In addition, most woodpeckers forage for their food of invertebrates by drilling and excavating into the bark and wood of dead and living trees. Many other birds nest in the abandoned cavities made by woodpeckers, or they use natural cavities formed in rotten parts of trees. Some other birds nest in or beneath woody debris, or they build platform nests on tall snags or living trees with a damaged top.
Because so many birds depend on cavities, maintaining this habitat feature has become an important consideration in forest management. The issue is especially prominent in the older-growth forests of the West Coast, where as many as six species of woodpeckers may co-occur in relatively large populations, along with many other cavity-dependent birds. If we wish these species to remain in regions where forestry is being practised, a substantial part of any management area must be maintained as mature or old-growth forest. If plantations are established, they should be designed to provide habitat for birds that require snags and dead logs. For example, cavity trees can be left standing during the harvest. Cavity- and snag-dependent species are also better accommodated by less intensive harvesting methods, such as selection cuts.
Image 23.4. Snags, or standing dead trees, are a critical habitat element for many animals. This photo shows four young kestrels (Falco sparverius) that recently fledged from a nest in a natural cavity in a pine snag left in a clear-cut in Nova Scotia. Source: I.A. McLaren.

Freshwater Biota
Forestry practices can degrade freshwater habitats in four major ways:
- by siltation (the settling of soil eroded from the land and streambanks)
- by increases in water temperature caused by the removal of shading vegetation from stream edges
- by blocking stream channels with logging debris
- by changes in hydrology
Damage may also be caused by accidental spills of fuel and as a result of pesticide spraying (see Chapter 22). Any of these assaults on freshwater habitat can affect populations of fish, amphibians, and aquatic invertebrates. These problems are especially severe in hilly or mountainous terrain because of the many small streams and rivers that occur there, and because steep slopes are highly vulnerable to erosion.
In most cases, it is possible to avoid or mitigate many of the damages caused to aquatic ecosystems. As we previously noted, erosion can be greatly reduced if roads and culverts are constructed carefully, logs are hauled correctly, and riparian buffers of uncut forest are left beside water-courses. Leaving buffer strips also avoids an accumulation of logging debris in streams, as does not felling trees into aquatic habitats. Riparian buffers are also effective at preventing increases in water temperature, because they shade streams even if the nearby forest has been harvested.

Old-Growth Forest
Old-growth forest is a late-successional (or climax) ecosystem that is characterized by at least some old trees, a multi-aged population (all age classes are represented, from young to old), and a complex physical structure. The structure of the habitat includes multiple layers within the canopy, some massive trees, large snags, and big logs lying on the forest floor. In some ecological contexts, the term “old-growth” is also used to refer to senescent populations of shorter-lived trees, such as older stands of poplar, birch, or cherry. This is not, however, the meaning of “old-growth forest” that is considered here.
Old-growth forest is a natural ecosystem with special values that are not replicated in plantations or even in natural regenerated secondary forests. For this reason, old-growth forest has great intrinsic value and is an important component of natural heritage. It also supports certain plants and animals that do not occur in other Canadian habitats. (However, this is a relatively minor attribute of temperate and boreal old-growth forest –tropical old-growth sustains enormously larger numbers of dependent species; see Chapters 7 and 26.) In addition, old-growth forest delivers important ecological services, such as storing carbon and providing clean water and air, and it has economic value for outdoor recreation and ecotourism.
Old-growth forest was once much more extensive in Canada (and elsewhere in the world) than it is today. In eastern Canada, for example, early “development” of the land by European settlers involved the extensive clearing of old-growth forest into agricultural land-uses, as well as its conversion into younger second-growth forest by timber harvesting. As a result, there is little of this ecosystem type left in the eastern provinces, where only a few percent of the total forest is now in an old-growth condition.
Old-growth forest is more abundant in parts of western Canada, particularly on the Pacific coast, where the wet climate favours the development of this natural ecosystem (because wildfires are uncommon). Even in British Columbia, however, extensive tracts of old-growth have been logged or converted to urbanized land-uses, especially near Vancouver and Victoria. And because old-growth timber is such a valuable resource, much of the remaining older forest is threatened by harvesting. It is likely that virtually all of the remaining old-growth forest will be logged during the next several decades and converted into second-growth forest, except where tracts are protected in ecological reserves and parks.
Many of the characteristics of old-growth forest, including elements of biodiversity, can be accommodated by so-called “new forestry” harvesting systems that are relatively “soft” in the intensity of disturbance that they cause. The best example of a new forestry system is selection cutting with retention of snags and cavity-trees. Because only some of the valuable timber is removed during a selection harvest, the physical and ecological integrity of the forest are left substantially intact, which conserves many of the old-growth values.
However, there are limits to what can be achieved through the new-forestry practices. If a goal of society is to preserve old-growth forest as a special kind of natural ecosystem, this is best done by establishing large, landscape-scale protected areas. The size of the protected areas is a critical factor because they must be big enough to sustain the long-term ecological dynamics that permit old-growth forest to develop, especially the natural disturbance regime. This landscape perspective is important because particular stands of old-growth forest cannot be preserved forever – they will inevitably become degraded by natural disturbance and/or environmental change. Consequently, old-growth forest can be sustained only if large protected areas are designated to preserve the necessary ecological dynamics.
Old-growth forest is an extremely valuable natural resource, more so than any other kind of forest. This is because it contains large individuals of economically desirable tree species. This timber can be used to manufacture valuable products, such as fine-grained, large-dimension lumber and plywood. However, stands of old-growth forest are rarely managed by foresters to maintain their defining characteristics. Rather, they are “mined” by harvesting, followed by silvicultural management to convert the site into a younger, second-growth forest. The second-growth forest will only be allowed to develop into a middle-aged forest before it is again harvested.
There is an economic rationale for this kind of management strategy. In the old-growth condition, a forest does not sustain a positive net production of biomass. This happens because, at the stand level, the productivity by living trees is more-or-less balanced by the deaths of other individuals through disease, accident, or old age. If the objective of management is to optimize the productivity of tree biomass, it is better to harvest a middle-aged forest soon after its net productivity begins to decrease, which is well before its attains an old-growth condition. Of course, this kind of economic thinking does not take account of the special ecological and aesthetic values of old-growth forest, which are degraded by logging.
Because old-growth forest has particular characteristics, some species of wildlife will only occur in this habitat. This is especially true of tropical old-growth forest. In Canada, animals that depend substantially on old-growth forest include the marbled murrelet (Brachyramphus marmoratus), the northern spotted owl (Strix occidentalis caurina), and the American marten (Martes americana). In addition, some plants are more abundant in old-growth than in younger, mature forest. Examples include the Pacific yew (Taxus brevifolia) and lungwort lichen (Lobaria pulmonaria). Ongoing studies will discover additional examples, especially of insects, lichens, mosses, and other less conspicuous elements of forest biodiversity. These indigenous biodiversity values are endangered by the continued logging of old-growth forest in Canada.
Canadian Focus 23.1. Controversy in Clayoquot Sound Clayoquot Sound is a Pacific embayment that reaches inland in central Vancouver Island to encompass a watershed of about 2,630 km2. The mountainous terrain supports a great variety of habitats, including extensive forest on the interior mountains and the flatter coastal plain. The climate is mild temperate, with abundant rainfall. The wet climate encourages the natural development of old-growth rainforest. This is a relatively uncommon ecosystem because few places have the conditions necessary for its development. Before commercial timber harvesting began, most of the Clayoquot forest was old-growth, with many trees being large and ancient – some >3 m in diameter and more than 1,000 years old. These old-growth trees have fine-grained wood because of their slow growth rate, which makes them extremely valuable as lumber.
Because of the extensive logging of old-growth forest in Clayoquot Sound (and elsewhere), this ecosystem is much less extensive today than it used to be. This is particularly true of the coastal plain and lower elevations in the mountains, where the most accessible forest occurred. Today, about 80% of the remaining forest occurs at higher elevations and on steeper slopes.
Almost all of the old-growth stands that are logged on Vancouver Island and elsewhere in coastal British Columbia regenerate to another forest. Moreover, the second-growth forest is dominated by the same species of trees as occurred in the original old-growth stands. However, the second-growth forest is harvested soon after its trees are large enough to be used to manufacture lumber or pulp, and this happens at a much younger age than is required to re-develop an old-growth condition. Much controversy has arisen over the rapid and extensive conversion of old-growth into second-growth forest. Many people believe that old-growth forest has great intrinsic value because it is a distinct natural ecosystem, and it is also valued for cultural, aesthetic, and ecological reasons.
Environmentalists have targeted the remaining old-growth forest of the Clayoquot area for protection, and over the years have focused much activism to that end. In part, this has occurred because the area is accessible and traversed by many people travelling to Pacific Rim National Park and the tourism area of Tofino. In fact, there are more extensive tracts of old-growth forest elsewhere on Vancouver Island and on the mainland of British Columbia. However, because those other forests are remote, protests there would have been less effective in attracting media and public attention than actions in the Clayoquot region.
The most intense protests began in March 1993, soon after the government of B.C. purchased stock in a company that had a licence to harvest timber in the Clayoquot region. A few weeks later, the government issued permits to log 74% of the area. This sparked an explosion of public demonstrations, including a large gathering at Clayoquot Sound. Some of the attendees had travelled across Canada by train, as part of a media event that symbolically began in Newfoundland and ended in coastal British Columbia. In addition to the protests and publicity stunts, there were blockades of logging roads and other kinds of civil disobedience, leading to the arrest of more than 850 people.
Image 23.6. This landscape in the Clayoquot Sound region was once covered mainly by old-growth rainforest. Because the valley floor supported the largest trees and was accessible, it was harvested first, in this case about 15 years prior to this photo being taken. The clear-cuts regenerate well through planted seedlings and natural seeding-in, and another mixed-species forest will again develop. However, this secondary forest will be harvested before it re-attains an old-growth condition. The stands at mid-slope were clear-cut three years before the photo, while the upper slopes have not yet been harvested. Source: B. Freedman. 
In October 1993, the premier of B.C. announced the establishment of a Scientific Panel for Sustainable Ecosystem Management in Clayoquot Sound. The panel was made up of 23 members, including experts in ecology, biodiversity, forestry, ecotourism, and other relevant interests. The mandate of this highly regarded group was to “make forest practice in Clayoquot not only the best in the province, but the best in the world.”
In June 1995, after a series of public meetings and other deliberations, the panel released a report containing 120 recommendations. These were accepted by the government and passed into law. The panel recommended that sustainable ecosystem management should be the over-riding objective for the Clayoquot region, and that all activities, including those of forestry, should be conducted with that objective in mind.
Many of the recommendations advocated silvicultural systems that would retain the key characteristics of old-growth rainforest, such as an uneven age structure, snags and cavity trees, woody debris, and healthy aquatic ecosystems. The panel felt that those objectives could be largely met by restricting the proportion of any large watershed that could be converted into younger age classes, accomplished by limiting the cut rate to no more than 1% of a catchment per year. That practice would ensure that sufficient areas of old-growth forest, or of managed forest having most of its habitat values, would always be present to satisfy the needs of dependent species. The panel also recommended that large areas be fully protected from forestry, particularly areas and sites of great value because of their ecological features, aesthetics, cultural significance, or utility for recreation.
The recommendations of the panel, and their acceptance by government, satisfied most people regarding the ecological sustainability of the forest-management plan for the Clayoquot Sound region. The panel’s work should not, however, be regarded as a perfect model of sustainability or as a permanent solution to the controversy over forestry in regions of old-growth forest.
Despite these positive developments, the controversy over logging the old-growth rainforest has not disappeared. Although logging companies and the British Columbia government committed to implementing the recommendations of the panel, the development of an ecosystem-based management plan did not happen in a timely fashion. Public protests again erupted over the continuation of relatively intensive logging within Clayoquot Sound, such as harvesting on steep slopes, the use of clear-cutting rather than selection-harvesting systems, harvesting at rates (within watersheds) greater than recommended by the panel, and failure to monitor effects of logging at the ecosystem level (Friends of Clayoquot Sound, 2014). New public protests were sparked by the resumption of road building and extensive logging on Catface Mountain. Some important lessons can be learned from this ongoing controversy:
- Scientists can recommend actions to make forestry more environmentally sustainable (this is true of all economic activities).
- The various interested parties may commit to undertake recommended changes, thereby alleviating the economic and political disruptions associated with public controversy.
- However, economic and political priorities may again shift, and thereby delay or prevent the implementation of changes.
- Therefore, citizens and environmentalists should always be skeptical about promises made by government and industry to protect environmental quality. Moreover, there is never a guarantee that a future government will not change agreed-upon rules (including legislated ones) in ways that provide less protection for the environment. It is even possible that the priorities of future generations of Canadians may support such an action.
The best hope of preventing future damage is to ensure that the social contract of forestry always includes an obligation to: (1) conduct industrial activities in a manner that does not degrade the timber resource, (2) maintain non-timber economic values such as hunted species and tourism, and (3) sustain the carbon-storage, hydrologic, and biodiversity values of old-growth forest and other elements of natural heritage. All of these forest values are important and must be maintained, and it is crucial that Canadians understand this. Unless this happens, the controversy will surely continue.
Plantations
The clear-cutting of natural forest is often followed by the planting of a new crop of trees, usually of a conifer species. These may then be intensively managed to increase the productivity of the stand. This system results in the development of a plantation (or tree-farm), which is an anthropogenic forest of a relatively simple character in comparison with the natural, mature or old-growth, mixed-species forest that previously occupied the site. Because many native species of the natural forest are unable to utilize the habitat available in a plantation, the ecological conversion has critical implications for biodiversity.
The most important habitat changes are related to differences in the tree species and physical structure of a plantation compared to natural forest. A typical plantation is dominated by trees of a particular species and of similar age and size (in population ecology this is known as a cohort, while in agriculture it is referred to as a monoculture). This is a greatly simplified ecosystem compared with natural forest, which typically has trees of various species, ages, and sizes. Such changes are greatest when the original forest is hardwood dominated, mixed hardwood-conifer, or old-growth. The changes are fewer if natural, even-aged conifer forest is replaced with a conifer plantation.

Of course, any changes in vegetation and habitat have secondary effects on the animals that can be sustained. Studies of conifer plantations in New Brunswick have found that they can support an abundant population of birds (Table 23.3). In fact, a 15-year-old plantation supported a larger bird population than did nearby natural forest, while species richness was similar. In this study, many birds of the natural, conifer-dominated forest began to invade the plantations once the trees were older than about 10 years.
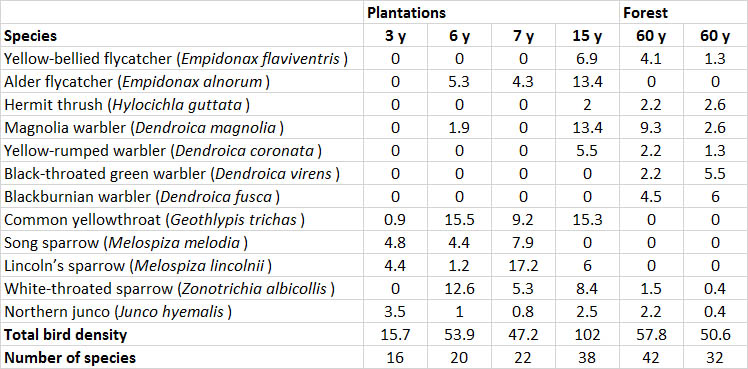
Plantations are especially deficient in cavity trees, snags, and woody debris. Consequently, they support few of the many species that require these habitat features. For example, in New Brunswick, conifer plantations are established by clear-cutting natural forest, then preparing the site for planting using large machines that crush the logging debris and topple any unharvested trees and snags. This intensive management results in the presence of almost no cavity trees or snags and little woody debris (Table
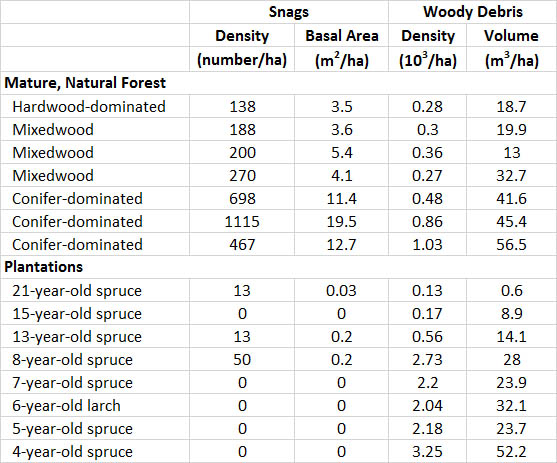
If conifer plantations provide adequate winter cover and browse, snowshoe hares (Lepus americanus) may be abundant. In fact, these animals may cause damage by feeding on the bark and shoots of young trees. As soon as the trees mature and start to produce sizable cone crops, red squirrels (Tamiasciurus hudsonicus) also find conifer plantations to be acceptable habitat.
Sometimes, plantations are established on previously agricultural or industrial lands (this is known as afforestation). Depending on the particular habitat that results, these plantations are likely to enhance the populations of some native birds by providing forest habitat. This can be a benefit in regions where agriculture is the dominant land-use, for example, in southern Ontario. However, greater biodiversity benefits would be attained if an attempt were made to restore a more natural forest, rather than a plantation.
Image 23.8. This 30-year-old plantation of white spruce (Picea glauca) in New Brunswick is a conifer forest, but it is simple in physical and biological structure. Although this habitat supports some native plants and animals, many others are eliminated by the scarcity of critical habitat elements, such as cavity trees and woody debris. Source: B. Freedman.

Landscape Considerations
Biodiversity at the level of landscape is related to the distribution and richness of ecological communities, including their dynamics over time (see Chapter 7). If a landscape is covered with only one or a few types of communities, it has little biodiversity at this level. In contrast, an area with a complex and dynamic mosaic of communities has much greater landscape-level biodiversity. Landscape ecology involves the study of the patterns and dynamics of communities on landscapes (and seascapes).
Landscape-level biodiversity is influenced by disturbances that result in some stands (or patches) of older communities being replaced with younger ones. Natural causes of these stand-replacing disturbances include wildfire, windstorms, volcanic eruptions, and insect irruptions. Anthropogenic causes include those associated with forestry.
Sometimes, timber harvesting is designed to mimic the natural patch-disturbance regime. For example, many pine forests are naturally disturbed by periodic wildfires, during which most of the mature trees may be killed. Soon afterward, a new cohort of tree seedlings establishes, and with time they grow into another mature forest. To some degree, foresters can emulate this natural disturbance regime when they develop plans to harvest and manage pine forest.
Forestry, by its nature, imposes an anthropogenic patch dynamic onto the landscape. This may occur if an un-natural mosaic of clear-cuts and plantations of various ages is created, interspersed in checkerboard fashion within a matrix of any remaining natural forest and non-forest habitats (such as wetlands). A landscape mosaic of this sort may even be recommended by game managers, because it can favour certain hunted species, such as deer and grouse. However, the patch dynamics created by forestry have important implications for many other elements of biodiversity. For example, if the remaining patches of natural forest are too small or isolated from each other, they will not sustain all of their native species and communities over the long term. These losses would have negative implications for the ecological sustainability of the forestry system (see Chapter 12).
Forestry creates fragmented landscapes that contain successionally dynamic patches of silvicultural and natural-forest habitats. Many species of native wildlife find the silvicultural habitats to be adequate for their purposes. However, such habitats and their dynamics are incapable of supporting other native elements of biodiversity, whose survival may therefore be at risk. If these native values are to be conserved, large patches of natural forest must be set aside as protected areas.
To achieve a balance between the needs of forestry and the responsibility to conserve indigenous biodiversity, consideration must be given to the size, shape, and spatial arrangement of the patches on managed landscapes, including the protected areas. For example, if the protected areas are too few, small, isolated, or young to accommodate all of their biodiversity objectives, then it will be necessary to design a landscape that is more ecologically appropriate. Design options that have been recommended to meet the biodiversity objectives of protected areas are examined in Chapter 26.
Some of the national parks of Canada are among the largest protected areas in the world. Yet many of these are too small to maintain viable populations of certain species, or to maintain the ecological dynamics required to allow old-growth forest to persist. Species that are most at risk need extensive areas of habitat to sustain their populations. They include the grizzly bear (Ursus arctos), wolf (Canis lupus), spotted owl, and marbled murrelet. Even the largest national parks may not be big enough to sustain these species over the centuries.
In such cases, the protected areas and their surrounding landscape must be managed as “greater protected areas” – as an integrated ecosystem. If forestry continues to be an important economic activity in the landscape around protected areas, it must be conducted with a view to sustaining those species and natural communities that might be at risk. In many cases, this will require changes in the forestry-management system. Such changes might include maintaining a network of protected areas that are connected by corridors and incorporate critical habitat elements, such as cavity trees and woody debris, into managed stands.
Global Focus 23.1. Is Canada “the Brazil of the North”? Sometimes, to gain an edge in the public sphere, individuals or organizations may use relatively extreme rhetoric when making arguments for or against an environmental position. For example, some opponents of intensive forestry practices have claimed that Canada should be viewed as “the Brazil of the North” because of the kinds of forestry being practised here. The point has been especially made in reference to the clear-cutting of old-growth coastal rainforest in British Columbia. But is it reasonable to assign a “Brazil of the North” label to Canada?
Obviously, Canada and Brazil are very different places in terms of people, culture, economy, and natural ecosystems. Nevertheless, there are key similarities with respect to forests and forestry between the two countries, as well as major differences. For example, although both countries are still heavily forested, each has lost a major part of the original forest to agricultural development and urbanization. In Brazil, the worst losses are of Atlantic tropical and subtropical forest, as well as forest in Amazonia. In Canada, it is mostly temperate forest in southern regions of the eastern provinces. Both countries have a large forest industry, although Canada’s is more export-oriented. Both countries have designated many protected areas (such as national parks), but neither has created enough such areas and their stewardship is deficient (for example, by not preventing damage caused by illegal logging or poaching of wildlife, controlling the damage caused by transportation corridors and tourism facilities, or protecting indigenous cultures).
A key ecological difference is in the biodiversity that is supported by the two countries. Canada has many indigenous species, but only a few are endemic (having a local distribution and occurring nowhere else). This is because Canada is a young country, in the ecological sense, having only been released from continental glaciation about ten-thousand years ago. In marked contrast, the natural ecosystems of Brazil are much older, and most have developed under subtropical and tropical climatic regimes. The humid forests of such regions support much higher levels of biodiversity, including many endemic species, compared with the natural ecosystems of Canada, which range from polar to temperate. In this vital respect, deforestation in Brazil causes enormously more grievous damage to biodiversity than it does in Canada because many more species and ecosystem types are affected, and the risks of causing extinctions are much greater. This context does not trivialize the importance of avoiding actions that cause native species or natural ecosystems of Canada to become at risk, but it is a valid comparison.
There are many additional comparisons that could be made when trying to understand whether we are the “Brazil of the North” or they are the “Canada of the South” (see selected data in table). Ultimately, however, subjective and extreme rhetoric is not particularly helpful when trying to help people develop informed opinions about important environmental issues. Table 23.5.
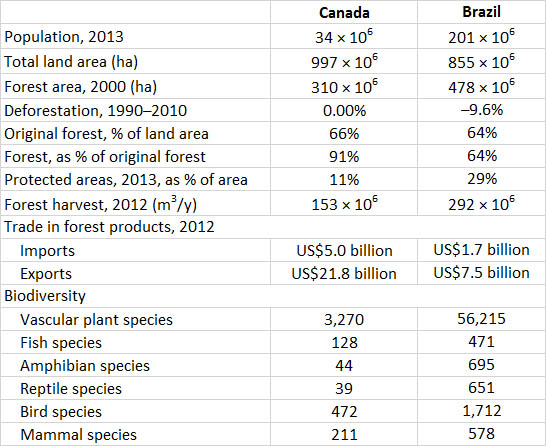
Sources: Data from World Resources Institute (2008), Chapter 14
Integrated Forest Management
Increasingly, foresters in many parts of Canada are working with other interested parties to develop integrated forest management plans that accommodate the need to harvest timber from forested landscapes while also sustaining other values. Usually, these plans focus on finding ways to conduct forestry while also supporting hunted species such as deer, elk, trout, and salmon. Efforts may also be made to accommodate other uses and values, such as non-consumptive recreation, for example, birding and hiking.
By co-operating in the design of integrated management plans, the forest industry is attempting to come to grips with some of the controversies that arise from their woodland operations. Society expects that the vast forests of Canada will continue to deliver a wide range of goods and services. These include the economic benefits from timber harvesting, while also satisfying the needs of sport hunters, fishers, hikers, and other outdoor recreationists. Even while they are used in these ways, forest landscapes are also expected to provide such ecologically important services as carbon storage, clean air and water, and to sustain native biodiversity.
The forest industry is making progress in the directions that society expects, but much more has to be done. This is particularly true of the need to set aside additional areas that are protected from forestry. If they are large enough, the protected areas can allow ecological processes to continue in a manner that is unfettered by major human stressors, so that natural ecosystems can develop and native species can sustain their populations. In addition, forestry will have to change to accommodate more of the habitat needs of biodiversity on managed sites.
If the Canadian forest industry is to legitimately claim that it is conducting its operations in an ecologically sustainable manner, it must achieve several broad objectives. First, it is critical that the rate of timber harvesting does not exceed that of forest productivity. At the same time, other economic values must be sustained, such as viable populations of hunted animals and opportunities for outdoor recreation. Finally, it is critical that no indigenous elements of biodiversity are made endangered by forestry. Although the forest industry has been making headway toward improving its environmental practices in Canada, not all of the requirements of ecologically sustainable forestry are being satisfied. Therefore, considerably more progress is required in this direction.
Conclusions
Forestry is a key economic sector in Canada, each year affecting millions of hectares of landscape. Timber harvesting and management of the subsequent regeneration cause many environmental changes, including decreases of carbon storage, alterations of hydrology, erosion, and effects on the habitat of wildlife. Because timber harvesting and silvicultural practices are severe disturbances of forested sites, some environmental damage is inevitable. To a large degree, however, many of the damages could be mitigated by adopting different forestry practices than are currently used. These include less use of the predominant clear-cutting system, and replacing it with softer practices such as selection harvesting. Greater attention to the protection of aquatic habitat is also necessary, such as retaining buffer strips of uncut forest along all watercourses. Some damage to the habitat of wildlife on cutovers can also be mitigated, for instance, by retaining cavity trees and by greater reliance on natural regeneration rather than on plantations. It is also necessary to protect large areas of natural forest from intensive resource harvesting. This requires the implementation of a connected network of protected areas that is sufficient to conserve those species and ecological communities that are incompatible with use of the landscape for forestry purposes.
Questions for Review
- How do forestry practices threaten the nutrient capital and site quality of harvested stands?
- How does timber harvesting affect the hydrology of streams and rivers?
- What elements should an integrated management plan include for a typical forested watershed in your region? Consider the needs to ensure a constant supply of timber, deer, sportfish, clean water, and habitat for non-game species.
- What are the characteristics of a typical old-growth forest in the region where you live? Do you think that the special values of the old-growth forest can be accommodated by forestry, or can old-growth forest be preserved only by creating large protected areas where trees are not harvested? What are the economic implications of setting aside such large tracts of potentially valuable timber? What are the ecological implications?
Questions for Discussion
- Consider a typical forest in the region where you live. What are the dominant species of trees, other plants, and animals that live in that forest? What are some important interactions among those species? Consider, for example, the habitat needs of certain animals, including their foods.
- Are forest products important in your life and in the functioning of your community? Compile a list that shows how trees are used for bio-energy, lumber, paper, and other products. Also consider non-timber uses of forests, such as hunted animals, recreation, carbon storage, and the provision of clean air and water.
- How might forest resources make a larger contribution to the Canadian economy, or to that of your region? Would it be possible, for example, to harvest more wood without degrading the timber resource? Could more people be employed in woodland operations if harvesting and management activities were less mechanized? Consider also the prospects for reducing the export of raw materials, such as logs, through increased local processing into manufactured products.
- Why is it essential that a large and connected system of protected areas be a key part of any landscape-scale plan for ecologically sustainable forestry?
Exploring Issues
- A proposal is being made to build a lumber mill in an area that is now wilderness. About 40% of the forest in the area is in an old-growth condition. You are an ecologist, working as part of a team of scientists to assess the potential environmental impacts of the forest management plan to supply timber to the proposed sawmill. Your responsibility is to consider the sustainability of the supply of timber and other forest resources, as well as effects on rare species and natural ecosystems. What would you examine to ensure that the forest management is ecologically sustainable?
References Cited and Further Reading
Barnes, B.V., S. Spurr, D. Zak, and S. Denton. 1998. Forest Ecology. 4th ed. John Wiley & Sons, New York, NY.
Berger, J.J. 1998. Understanding Forests. Sierra Club, San Francisco, CA.
Bormann, F.H. and G.E. Likens. 1994. Pattern and Process in a Forested Ecosystem. Springer, New York, NY.
Boyce, M.S. and A. Haney (eds.). 1999. Ecosystem Management: Applications for Sustainable Forest and Wildlife Resources. Yale University Press, New Haven, CT.
Buskirk, S.W. 1992. Conserving circumboreal forests for martens and fisher. Conservation Biology, 6: 318-320.
Crowell, M. and B. Freedman. 1994. Vegetation development during a post-clearcutting chronosequence of hardwood forest in Nova Scotia, Canada. Canadian Journal of Forest Research, 24: 260-271.
Drolet, C.A. 1978. Use of forest clear-cuts by white-tailed deer in southern New Brunswick and central Nova Scotia. Canadian Field-Naturalist, 92: 275-282.
Freedman, B. 1995. Environmental Ecology. 2nd ed. Academic Press, San Diego, CA.
Freedman, B., R. Morash, and A.J. Hanson. 1981. Biomass and nutrient removals by conventional and whole-tree clear-cutting of a red spruce-balsam fir stand in central Nova Scotia. Canadian Journal of Forest Research, 11: 249-257.
Freedman, B., C. Stewart, and U. Prager. 1985. Patterns of Water Chemistry of Four Drainage Basins in Central Nova Scotia. Technical Report IWD-AR-WQB-85-93, Environment Canada Moncton, NB.
Freedman, B., S. Woodley, and J. Loo. 1994. Forestry practices and biodiversity, with particular reference to the Maritime Provinces of eastern Canada. Environmental Reviews, 2: 33-77.
Freedman, B., V. Zelazny, D. Beaudette, T. Fleming, S. Flemming, G. Forbes, G. Johnson, and S. Woodley. 1996. Biodiversity implications of changes in the quantity of dead organic matter in managed forests. Environmental Reviews, 4: 238-265.
Friends of Clayoquot Sound. 2014. About Clayoquot Sound. www.focs.ca/ Gillis, A.M. 1990. The new forestry: an ecosystem approach to land management. BioScience, 40: 558-562.
Gullion, G.W. 1988. Aspen management for ruffed grouse. Pp. 9-12 in: Integrating Forest Management for Wildlife and Fish. General Technical Report NC-122, North Central Forest Experiment Station, St. Paul, MN.
Harris, L.D. 1984. The Fragmented Forest. University of Chicago Press, Chicago, IL.
Hunter, M.L. 2003. Wildlife, Forests, and Forestry: Principles of Managing Forests for Biological Diversity. Prentice Hal0,l Englewood Cliffs, NJ.
Johnson, G.A.M. and B. Freedman. 2002. Breeding birds in forestry plantations and natural forest in the vicinity of Fundy National Park, New Brunswick. Canadian Field-Naturalist, 116: 475-487.
Kimmins, J.P. 2003. Forest Ecology: A Foundation for Sustainable Management. 2nd ed. Upper Saddle River, NJ: Prentice Hall College Division.
Kimmins, J.P. 2005. Forest Ecology. 3rd ed. Macmillan, New York, NY.
Kimmins, J.P. 1997. Balancing Act: Environmental Issues in Forestry. 2nd ed. University of British Columbia Press, Vancouver, BC.
Krause, H.H. 1982. Nitrate formation and movement before and after clear-cutting of a monitored watershed in central New Brunswick. Canadian Journal of Forest Research, 12: 922-930.
Likens, G.E. and F.H. Bormann. 1999. Biogeochemistry of a Forested Ecosystem. 2nd ed. Springer, New York, NY.
Martin, C.W., R.S. Pierce, G.E. Likens, and F.H. Bormann. 1986. Clearcutting Affects Stream Chemistry in the White Mountains of New Hampshire. NE-579, Northeastern Forest Experiment Station, USDA Forest Service, Broomall, PA.
Maser, C. 1990. The Redesigned Forest. Stoddart, Toronto.
Maser, C. 2001. Forest Primeval: The Natural History of an Ancient Forest. Oregon State University Press, Corvallis, OR.
Maser, C., A.W. Claridge, and J.M. Trappe. 2007. Trees, Truffles and Beasts: How Forests Function. Rutgers University Press, Rutgers, NJ.
McRae, D.J., L.C. Duchesne, B. Freedman, T.J. Lynham, and S. Woodley. 2001. Differences between wildfire and clearcutting and their implications in forest management. Environmental Reviews, 9: 223-260.
Morgan, K. and B. Freedman. 1986. Breeding bird communities in a hardwood forest succession in Nova Scotia. Canadian Field-Naturalist, 100: 506-519.
Smith, D.M., B.C. Larson, M.J. Kelty, and P.M.S. Ashton. 1996. The Practice of Silviculture: Applied Forest Ecology, 9th Ed. J. Wiley and Sons, New York, NY.
Swan, D., B. Freedman, and T. Dilworth. 1984. Effects of various hardwood forest management practices on small mammals in Nova Scotia. Canadian Field-Naturalist, 98: 362-364.
Swanson, F.J. and J.F. Franklin. 1992. New forestry principles from ecosystem analysis of Pacific Northwest forests. Ecological Applications, 2: 262-274.
Welsh, D. and D.R. Fillman. 1980. The impact of forest cutting on boreal bird populations. American Birds, 34: 84-94.
World Resources Institute. 2008. Earth Trends. The Environmental Information Portal. Washington, DC. http://www.wri.org/


