27: The Biodiversity Crisis
- Page ID
- 42052
Learning Objectives
After completing this chapter, you will be able to
- Outline how humans are causing the modern crisis of extinction and endangerment.
- Give examples of species that have been made extinct through human activities, including cases from Canada.
- Explain how a system of protected areas is essential to the preservation of biodiversity.
- Outline the roles of governments, non-governmental organizations, scientists, and citizens in conserving species and other elements of biodiversity.
Introduction
Earlier, we defined biodiversity as the total richness of biological variation and examined reasons why it is important (see Chapter 7). In this chapter, we examine the many threats to biodiversity that are associated with the human economy. The emphasis is on severe damage that is being caused, especially that associated with losses of species and of natural ecosystems.
Extinction refers to the loss of a species or another named biological entity (referred to as a taxon) over all of its range on Earth. Extirpation is a more local disappearance, with the taxon surviving elsewhere. Extinction represents an irretrievable loss of a unique portion of the biological richness of Earth, whereas it may be possible to re-establish an extirpated taxon from a surviving population.
Extinctions have always occurred as a result of natural influences. These include random catastrophes as well as the longer-term effects of environmental change, such as in climate or in biological factors such as disease or predation (Chapter 7). In modern times, however, and even for the past 10-thousand years or so, almost all extinctions have been caused by anthropogenic influences, particularly by over-harvesting and the destruction of natural habitats. In fact, an enormous increase in the rate of extinctions has been one of the most important consequences of humans becoming the dominant species on Earth. Species are now disappearing so quickly that we refer to the phenomenon as an extinction crisis (or a biodiversity crisis).
Every species is unique, a fact that gives them great intrinsic value – this is a central tenet of the biocentric world view (see Chapter 1). Therefore, from an ethical perspective, any irretrievable loss of biodiversity is a shameful consequence of the ways that people are using their power to exploit other species and ecosystems. This is also a foolish way for humans to administer their global empowerment, because unique species are disappearing before they have been investigated for their potential usefulness in medicine or agriculture, and before we understand their importance as components of ecosystems. Human actions that result in extinctions can only be regarded as ecologically dangerous behaviour.
In this chapter, we examine the modern biodiversity crisis – the reasons why it is happening, ways of repairing at least some of the damage already caused, and how to prevent further losses.
Natural Extinctions
Life has existed on Earth for about 3.5 billion years. Almost all of the species that have lived during that period of time are now extinct, having disappeared “naturally” for some reason or other. (The survivors are referred to as extant, or still living today.) In many cases, the extinct species could not adapt to changes that occurred in their environment, such as shifts in climate or increases in disease, predation, or competition with other species. Many species, however, disappeared during brief episodes of mass extinction, which may have been caused by unpredictable catastrophes, such as a meteorite hitting Earth.
The geological record clearly shows that many species and groups of organisms (such as genera, families, and phyla) have appeared and disappeared over time (see Chapter 6). For example, many phyla of invertebrate animals evolved relatively quickly during an evolutionary proliferation that occurred around the beginning of the Cambrian era, about 570 million years ago. Subsequently, most of those phyla and their many species became extinct. About 20 extinct phyla from that period were discovered in a renowned fossil deposit known as the Burgess Shale, located in Yoho National Park in southeastern British Columbia. In an evolutionary sense, each of those extinct phyla represented a novel “experiment” in the form and function of invertebrate animals. We only know that these ancient creatures existed because their fragile body structures became fossilized under extraordinary geological circumstances (Gould, 1989).
The fossil record is replete with many other examples of ancient extinctions. However, the rate of extinction, and of the subsequent evolutionary radiation of new species, has not been uniform over time. The geological record clearly shows that relatively low and uniform rates of extinction have typically persisted for extremely long periods of time, but those tranquil eras were punctuated by about nine catastrophic events of mass extinction.
The most intense event occurred at the end of the Permian era, about 245 million years ago. This natural catastrophe resulted in the loss of about 54% of the existing families of marine animals, including 84% of the genera and 96% of the species (Erwin, 1990). Another mass extinction occurred at the end of the Cretaceous period, about 65 million years ago. This famous event involved the last of the dinosaurs and pterosaurs (flying reptiles), along with many other taxa, totaling perhaps three-quarters of the species living at the time. Many scientists believe that this crisis of paleobiodiversity was caused by a meteorite hitting the Earth, likely in the vicinity of the Yucatan peninsula of Mexico. Such a catastrophe would have caused a deadly tsunami, while also ejecting enormous quantities of dust into the atmosphere, likely resulting in a cooling of the climate that most species were unable to tolerate.
During the past several centuries, Earth’s existing heritage of biodiversity has been buffeted by another mass extinction. This is an ongoing catastrophe, and it will certainly intensify into the foreseeable future. This ecological calamity is not a natural phenomenon. Rather, it is being caused by the influences and economic activities of modern humans.
Extinctions Caused by Humans
Many kinds of human activities are causing species to become endangered or extinct. (A species is considered endangered if, because of a small population or loss of habitat, it has a high risk of becoming extirpated or extinct.) The most important cause is the destruction of natural ecosystems and their conversion into habitats that are unsuitable for the original species, a problem that is especially acute in tropical countries. Excessive harvesting of some species is also significant, as is damage caused by introduced predators, diseases, and competitors. Any of these stressors can cause populations to become increasingly small and fragmented, a circumstance that results in much greater risks of extirpation or extinction (Figure 27.1).
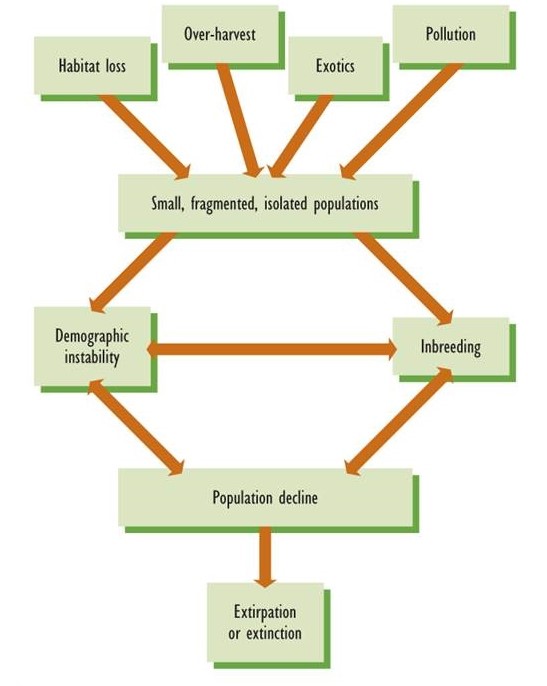
Because of these anthropogenic influences, the past several centuries have witnessed huge increases in the global rate of extinction and in the number of species that are threatened with this catastrophe. Not surprisingly, our knowledge of recently extinct and endangered species is relatively complete for large and conspicuous species such as vertebrate animals, especially those that live in temperate and higher-latitude countries, where most biologists also live.
In fact, there have been at least 842 known extinctions, including 746 animals and 96 plants (many of these are distinct subspecies and varieties; IUCN, 2014). The losses include 71 species of mammals, 135 birds, 22 reptiles, 34 amphibians, and 80 fishes. All of these extinctions were caused by human influences. Much larger numbers of species are at grave risk of suffering extinction (1,199 species of mammals are at risk, as are 1,373 birds, 927 reptiles, 1,957 amphibians, and 2,222 fishes).
Unfortunately, we know much less about extinctions among less conspicuous groups of organisms. This is particularly true of the enormous diversity of relatively small, poorly known species that live in tropical ecosystems, especially in old-growth rainforest. Undoubtedly, huge numbers of tropical species, particularly plants and invertebrates, have become extinct during the past several centuries as their natural forest habitat was converted into agricultural and other land-uses. We can refer to these losses as “hidden extinctions” because so few of the lost species had been discovered and named by taxonomists. Moreover, these hidden extinctions continue to occur rapidly, in fact at an accelerating rate, because their poorly explored tropical habitats are being destroyed so quickly.
In the following sections we will examine selected case studies of species that have been rendered extinct by humans and their activities.
Prehistoric Extinctions
Many species are useful as “resources” that humans can harvest and use as a source of food, medicine, timber, fuel, or some other purpose. In many cases, the exploitation of these potentially renewable resources has been so insatiable that their “mining” has culminated in extinction. These once-valuable species now occur nowhere on Earth (Freedman, 1995).
We previously examined extinctions that were caused by prehistoric hunters as they over-hunted populations of large, naive animals in newly discovered lands (see Global Focus 12.2). In North America, it appears that paleohunters exterminated many species of large mammals soon after people discovered the continent by migrating across a Beringean land bridge more than 12 millennia ago, at the end of the most recent ice age. Known extinctions occurring around that time include 77 species of mammals, such as 10 species of horse, a giant ground sloth, four kinds of camels, two bison, the mastodon, several mammoths, and the sabre-toothed tiger. Other large animals became extinct when South America was colonized somewhat later.
The colonization of “new” land masses by humans also caused prehistoric mass extinctions in other places. Australia and New Guinea were discovered about 50-thousand years ago. Soon after, many species of marsupials, large flightless birds, and tortoises became extinct, likely because of over-hunting.
New Zealand was colonized less than one-thousand years ago. Within two centuries, 30 large birds were extinct, among them a goose, a swan, and 26 species of large flightless birds known as moas. The extinctions proceeded as an anthropogenic wave that began in northern North Island, which was the initial point of colonization, to southern South Island. Many of the moas were herded by the hunters and their dogs to convenient butchering sites, where the great piles of bones were later used by early European farmers as a source of phosphate fertilizer.
In a similar fashion, the human colonization of Madagascar, about 1,500 years ago, resulted in the extinction of 14 species of lemurs, 6-12 flightless elephant birds, and various other big and edible animals. Other well-known prehistoric mass extinctions occurred on Hawaii, New Caledonia, Fiji, and the West Indies. In fact, this phenomenon likely occurred whenever a previously inhabited island was discovered and colonized.
The endemic (local) species that existed only on small islands are particularly vulnerable to extinction. The key reasons for this were:
- island species occur in small, isolated populations, which are especially vulnerable to extinction
- many birds of remote islands had not experienced intense predation during their recent history, and as a result they had evolved to be flightless, relatively large, and unafraid of predators, so they were extremely vulnerable to hunting by people once their islands were colonized
- most island species did not co-occur with closely competing organisms, so they were easily displaced when more capable species were introduced
- islands also became ecologically degraded by introduced plants, animals, and diseases
- finally, the human colonization of remote islands, particularly by Europeans, resulted in extensive destruction of natural habitats as the islands were cleared for agricultural, urban, and tourism developments.
For these reasons, the species of remote islands have suffered particularly high rates of extinction. For example, at one time, each of the approximately 800 islands of the southern Pacific Ocean may have had several endemic species of flightless rails (a family of marsh birds known as Rallidae), plus other unique birds and reptiles. As these islands were discovered and colonized by prehistoric Polynesians, perhaps thousands of these endemic species became extinct through over-hunting and habitat damage. For instance, a study of bird bones recovered at an archaeological site on the island of Ua Huka found that 14 of the 16 original birds no longer occur there, including 10 endemic species that had been rendered extinct. The extinctions worsened when Europeans secondarily colonized these and other oceanic islands, because of the extensive habitat losses that occurred during “development.” In fact, of the 135 taxa of birds (including 95 species) around the world that are known to have become extinct since 1600, all but nine lived on islands.
The problem of extinction-prone island biotas can be further illustrated by the case of the Hawaiian Islands, an ancient, remote archipelago of volcanic outcroppings in the central Pacific Ocean. When these islands were first discovered by Polynesian seafarers, there were at least 86 species of birds, including 68 that occurred nowhere else. Of those 68 endemics, 24 are now extinct and 29 are endangered. Similarly, the native flora at the time consisted of as many as two thousand species of flowering (angiosperm) plants, of which as many as 98% were endemic. During the past several hundred years, more than 100 of the endemic plants became extinct, and more than 500 are threatened or endangered. The extinction and endangerment of Hawaiian species has been caused by the widespread conversion of natural habitats into agricultural and urbanized land-uses, coupled with introductions of alien predators, competitors, virulent diseases, and destructive herbivores such as goats.
Historic Losses by Over-Harvesting
Unsustainable harvesting during historic times has caused some of the most famous cases of extinction and endangerment, in some instances involving species that were initially extremely abundant. We will illustrate this phenomenon by referring to the dodo, great auk, passenger pigeon, and other notable cases. These are examples of the devastating effects that insatiable killing can have on vulnerable populations of wild creatures.
The dodo (Raphus cucullatus) was a turkey-sized, flightless bird that disappeared in 1681, making it the first documented extinction of the historical era. The loss of this species is immortalized in everyday language by the phrase “dead as a dodo,” which is used as a metaphor for an irrevocable loss. The word “dodo” is also sometimes used to indicate an old-fashioned or stupid person. This etymology derives from the dodo’s apparent inability to adapt to threats posed by the human colonists of Mauritius, the only place where this bird lived. Mauritius is a small island in the Indian Ocean, discovered by Portuguese sailors in 1507. In 1598 it was colonized by the Dutch, who hunted the dodo for meat, gathered its eggs, and cleared its habitat for agriculture. They also released cats, pigs, and monkeys that preyed on dodos and destroyed their ground-level nests. These stressors caused the dodo to decline rapidly and become extinct.
The great auk (Pinguinus impennis) , a flightless seabird, was the first documented anthropogenic extinction of a species whose range included North America. Early mariners knew it as the original “pennegoin,” although it belonged to a different family of birds (Alcidae) than the outwardly similar penguins (Spheniscidae) of the Southern Hemisphere. The great auk lived throughout the north Atlantic region, breeding on a few islands off eastern Newfoundland, in the Gulf of St. Lawrence, around Iceland, and north of Scotland. This large seabird was initially abundant in its breeding colonies. Because it was flightless, it could be easily killed. Consequently, the great auk had long been exploited by Aboriginal people inhabiting what is now known as Newfoundland, and also by Icelanders and European fishers, as a source of meat, eggs, and oil. Unfortunately, the great auk developed into a valuable commodity when its feathers became sought after for stuffing mattresses in the mid-1700s. This resulted in a relentless slaughter that quickly caused the great auk to become extinct.
One of the largest breeding colonies of great auks was on Funk Island off eastern Newfoundland. In 1785, an observer described the harvest of great auks and other seabirds on Funk Island (Nettleship and Evans, 1985):
“It has been customary of late years, for several crews of men to live all summer on that island, for the sole purpose of killing birds for the sake of their feathers, the destruction of which they have made is incredible. If a stop is not soon put to that practice, the whole breed will be diminished to almost nothing, particularly the penguins.”
The great auk was, in fact, extirpated from Funk Island in the early 1800s. The last two individuals seen alive were killed in 1844 by several Icelanders who were searching for specimens to sell to a bird “collector” of natural-history specimens. Because of their extreme rarity at the time, great auks and their eggs were precious to collectors – they were, unfortunately, too valuable to let live.
The passenger pigeon (Ectopistes migratorius) may have numbered as many as 5-billion individuals three centuries ago, when it may have been the most populous landbird in the world. It bred in mature forests of oak, beech, hickory, and chestnut in southeastern Canada and the northeastern United States. These trees produce large seeds known as “mast,” which were a key food for this bird. In the autumn, passenger pigeons migrated in enormous flocks to the southeastern United States. Their immense flocks were described as being so dense as to obscure the sun, and taking hours to pass. The birds roosted communally during winter nights, often in such large numbers that they would kill trees by an excessive deposition of guano (bird feces), and would break stout limbs under their weight.
The naturalist John Lawson described an impressive passage of these birds in the Carolinas (Feduccia, 1985):
“I saw such prodigious flocks of these pigeons . . . in 1701-2 . . . that they had broke down the limbs of a great many trees all over these woods, whereupon they chanced to sit and roost . . . These pigeons, about sun-rise . . . would fly by us in such vast flocks, that they would be near a quarter of an hour, before they were all passed by; and as soon as that flock was gone, another would come; and so successively one after another, for the rest of the morning.”
The seemingly unlimited abundance of passenger pigeons, and their habit of migrating and breeding in large and dense groups, made them an easy target for market hunters who sold their carcasses in cities and towns. During the early 1800s there was a well-organized hunt of passenger pigeons to supply urban markets with cheap meat. During seasons when the hunt was on, “wagon loads of them . . . poured into the market . . . and pigeons became the order of the day at dinner, breakfast, and supper, until the very name became sickening” (A. Wilson in 1829; quoted in Feduccia, 1985).
The sizes of the harvests were staggering. For example, about one-billion pigeons were taken in 1869 in breeding colonies in Michigan alone. The intensity of the commercial hunting far exceeded sustainability, and this, along with destruction of much of the breeding habitat, caused the passenger pigeon to decline rapidly in abundance. The last known attempt at nesting was in 1894, and the last known individual died a lonely death in the Cincinnati Zoo in 1914.
The Carolina parakeet (Conuropsis carolinensis) once bred widely in the southeastern United States. This parakeet was a fairly common, brightly plumaged, fruit- and seed-eating bird that foraged and roosted in groups, especially in mature hardwood forest. Carolina parakeets were not hunted as a valuable commodity. Rather, they were exterminated because they were regarded as an agricultural pest, because of damage they caused while feeding in orchards and grain fields. Unfortunately, Carolina parakeets were an easy mark for eradication because they nested and fed communally. Also, they tended to assemble around wounded colleagues, which allowed an entire flock to be easily wiped out by a hunter. The last record of a flock of these parakeets was in 1904, and the last known individual died in a zoo in 1914.
The Steller’s sea cow (Hydrodamalis stelleri) was a mammal related to the manatees. It lived in subarctic waters around the Aleutian Islands in the Bering Sea and was hunted by Aboriginal people of that region. Soon after this shy and inoffensive species was “discovered” by Russian explorers in 1741, it was hunted as a source of food and hides and was rendered extinct after only 26 years of exploitation.
The Caribbean monk seal (Monachus tropicalis) lived in the Caribbean Sea and Gulf of Mexico. This species was encountered, and eaten, on the second voyage of Christopher Columbus to the Americas in 1494. Populations of this seal were depleted by an eighteenth-century market hunt for its meat and blubber. The last survivors were exterminated by the subsistence hunting of local fishers.
The Eskimo curlew (Numenius borealis) is a large sandpiper that was still abundant as recently as 150 years ago. It was exploited by market hunters during its migrations through the prairies and coasts of Canada and the United States, and also on its wintering grounds on the pampas (grasslands) and coasts of South America. The uncontrolled hunting caused this bird to become rare by the end of the nineteenth century. The last observed nesting attempt was in 1866, and the last specimen was “collected” (by shooting) in Labrador in 1922. For some decades the Eskimo curlew was thought to be extinct, very small numbers of this perilously endangered bird may have recently been seen by expert birders.
The right whale (Balaena glacialis) once ranged over all temperate waters of the Northern Hemisphere. Because if its rich oil content, habit of swimming at a relaxed speed in coastal waters, and the fact that it floated when dead, early whalers considered this the “right” whale to hunt. Due to commercial over-hunting of right whales for their blubber, which was rendered into oil to fuel the lamps of Europe and America, its populations collapsed over its entire range. This whale has been extirpated from the eastern Atlantic off Europe, and it is critically endangered in the western Pacific off Korea and Japan. Only about four hundred right whales survive in the northwest Atlantic Ocean. Most of these animals spend much of the summer and autumn in the mouth of the Bay of Fundy and off southwestern Nova Scotia. They migrate south to spend the winter along the southeastern United States and eastern Caribbean. Although not hunted for decades, the population of right whales has been slow to recover, largely because of mortality caused by collisions with ships and entanglement in fishing gear.
Image 27.1. The Labrador duck (Camptorhynchus labradorium) used to winter on the Atlantic coast of Canada and the United States and probably nested in Labrador. Because of excessive hunting, it became extinct around 1875. This is a photograph of carved models of a pair of Labrador ducks, replicated from old stuffed specimens that had been “collected” in Nova Scotia by a nineteenth-century naturalist. Source: B. Freedman.

Losses by Habitat Destruction
Many species have been rendered endangered or extinct because their natural habitats were converted to agricultural or other land-uses or were damaged by invasive alien species. We will first examine several examples of this phenomenon, and then assess the modern destruction of tropical forest, which is the human activity that is most important in causing extinctions today.
The American ivory-billed woodpecker (Campephilus principalis principalis) lived in the southeastern United States, where it bred in extensive tracts of mature, bottomland, hardwood forest and cypress swamp. Most of this habitat was heavily logged or converted to agriculture by the early 1900s, which drove the population of ivory-billed woodpeckers into a rapid decline. There had been no sightings of this woodpecker since the early 1960s, but astonishingly, in 2005, one individual was photographed in a remote forest tract in Arkansas, prompting hope that the species might yet be recovered. A closely related subspecies, the critically endangered Cuban ivory-billed woodpecker (Campephilus principalis bairdii), may still occur in tiny numbers in mountain forests in Cuba.
The black-footed ferret (Mustela nigripes) was first “discovered” in the prairies of North America in 1851. Because of habitat loss, this predator became extirpated in Canada and endangered in the United States. Extensive areas of its habitat of short-grass and mixed-grass prairie were converted into agricultural use. Also, its principal food, the prairie dog (Cynomys ludovicianus), has declined in abundance. The prairie dog has been relentlessly poisoned as a perceived pest of rangeland. With little habitat or food, the black-footed ferret is unable to survive over most of its former range. Nevertheless, a cooperative recovery program of the U.S. Fish and Wildlife Service and the Canadian Wildlife Service has resulted in the release of captive-bred ferrets into places where suitable habitat still exists, and they appear to be increasing in abundance in those places. A small population is now present in Grasslands National Park in southern Saskatchewan.
The Furbish’s lousewort (Pedicularis furbishiae) is an herbaceous plant that grows only along a 230-km stretch of the Saint John River valley in New Brunswick and Maine. This species had been considered extinct, but in 1976 it was “re-discovered” by a botanist doing field studies of the potential environmental impacts of a proposed hydroelectric reservoir on the upper Saint John River in Maine. That industrial development would have obliterated the only known habitat of the lousewort. For that, and other environmental and economic reasons, the dam was not constructed.
Canadian Focus 27.1. Alien Invaders During the past five centuries, and at an accelerating pace, Canada has become host to an enormous number of alien plants, animals, and microorganisms. Many of them were introduced intentionally, and others accidentally. Some have caused severe damage by invading natural ecosystems and displacing native species or by becoming serious predators or pathogens of native biota. Others are causing awful economic damage as pests in agriculture, forestry, horticulture, or in the home. Canada is not unique in this circumstance – all countries are suffering grave ecological and economic damage from invasive aliens. In fact, this syndrome is one of the biggest environmental problems facing the planet. There is a litany of examples of invasive aliens in Canada. The following cause some of the most important ecological damage: Invaders of Natural Habitats
- Garlic mustard (Alliaria petiolata) is a Eurasian plant that was accidentally introduced to America, possibly as a contaminant of crop seed or by hitchhiking in soil carried as ballast on sailing ships. This herbaceous plant invades bottomland forests of southern Ontario and Quebec, where it crowds out native plants, some of which are rare.
- Gorse (Ulex europaea) is a European shrub that was introduced as a horticultural plant. It is invasive in coastal British Columbia, where it displaces at-risk plants that live in dry forests of Garry oak (Quercus garryana) and Douglas-fir (Pseudotsuga menziesii).
- Purple loosestrife (Lythrum salicaria) is a herbaceous Eurasian plant that was introduced as an ornamental species or with ship ballast. It can degrade wetland habitat for native plants and animals.
- Leafy spurge (Euphorbia esula) was accidentally introduced as a contaminant of crop seed. It invades prairie and displaces rare native species.
- The brown spruce longhorn beetle (Tetropium fuscum) is a Eurasian insect that arrived in Halifax in the 1990s, probably carried in wood used to secure ship cargoes. It is attacking and killing native spruce trees, especially red spruce (Picea rubens), and it may be a threat to the entire boreal forest.
- Chestnut blight (Endothia parasitica) and Dutch elm disease (Ceratocystis ulmi) are Asian fungal pathogens that were brought to North America with horticultural stock of alien trees. These diseases have wiped out native chestnut and elms wherever encountered, causing terrible damage to natural forests. A similar recent case introduced through horticulture is the butternut canker (Sirococcus clavigignenti), which is now killing butternut trees (Juglans cinerea).
- The common carp (Cyprinus carpio) is a Eurasian fish introduced as a source of food and sport. It damages shallow-water habitats by uprooting aquatic plants and disturbing sediment while feeding and nesting.
- The zebra mussel (Dreissenia polymorpha) arrived to the Great Lakes in ballast water of ships from Europe. It causes damage by displacing native mollusks and by fouling water pipes and other structures.
- The green crab (Carcinus maenas) arrived in ballast water in the mid-nineteenth century and is now firmly established on the East Coast. It feeds broadly and has caused declines of many native invertebrates.
- The green fleece (Codium fragile) is a marine alga from Eurasia that recently (about 1990) established on the Atlantic and Pacific coasts. It displaces native seaweeds, particularly in the Atlantic. Invaders of Anthropogenic Ecosystems:
- The starling (Sturnus vulgaris), English sparrow (Passer domesticus), and rock pigeon (Columba livea) are Eurasian birds that were introduced to America by “homesick” European immigrants. They are now extremely abundant and displace native birds from breeding sites, compete with them for food, and foul urban areas with their excrement.
- The common rat (Rattus norvegicus), house mouse (Mus musculus), and cockroach (Blatta orientalis) are alien animals that are pests in many homes.
- The dandelion (Taraxacum officinale), crabgrass (Digitaria sanguinalis), and plantain (Plantago major) are among many alien plants that were introduced to North America, mostly by accident, and are now considered pests of horticulture.
- Bull thistle (Cirsium vulgare), groundsel (Senecio jacobea), and St. John’s wort (Hypericum perforatum) are among many invasive aliens that degrade pastures by crowding out more nutritious plants or by being distasteful or poisonous to livestock.
Tropical Deforestation
Tropical forest is most biodiverse ecosystem on Earth – its richness of species is unparalleled. Moreover, this poorly explored biome is thought to contain millions of as-yet-unnamed species, particularly of insects (Chapter 7). Because so many tropical-forest species have a local distribution, the destruction of this ecosystem causes a disproportionate number of extinctions (in comparison with those by the clearing of other kinds of natural ecosystems).
It is well known that the rate of deforestation in most tropical countries has increased alarmingly during the past century, particularly in the past several decades. This is in marked contrast to the situation in most higher-latitude countries, where forest cover has been relatively stable (see Chapter 14). In North America, for example, there has been little net change in forest cover in recent years (Table 27.1). In contrast, most countries of Central and South America had substantial losses of forest cover during that period, as did most tropical countries of Africa and Asia. Overall, the developing world lost 138 million hectare of forest between 1990 and 2005 (5.8% overall), and most of that was tropical forest (WRI, 2008). Globally, the rate of clearing of tropical rainforest during the 1980s and 1990s was equivalent to more than 1% of that biome per year—a rate that, if maintained, would imply a half-life for that biome of less than 70 years.
Most tropical deforestation is caused by the conversion of forest into subsistence agriculture by poor families. This agricultural conversion is greatly increased whenever access to the forest interior is improved. When roads are constructed for timber extraction or mineral exploration, deforestation often follows rapidly. The complex social causes of deforestation include population growth, inequality of land ownership, and the displacement of poor families by mechanization and the global commercialization of agriculture. Because of these factors, enormous numbers of poor families are seeking arable land in most of the less-developed countries. These people need land on which they can grow food for subsistence and for some cash income.
The forest conversion often involves a system of shifting cultivation, in which the trees are felled, the woody debris burned, and the land used to grow mixed crops for several years. By that time, fertility has declined and weeds have become abundant. The land is then abandoned for a fallow period of several decades. This allows a secondary forest to regenerate, while nearby patches of forest are cleared to provide new land for cultivation.
A more intensive system of subsistence agriculture, known as slash-and-burn, results in a permanent conversion of the land into crop production. Slash-and-burn also involves cutting and burning the forest. After the forest is gone, however, the land is used continuously, without a fallow period during which a secondary forest may regrow and site fertility regenerate.
Much tropical forest is also affected by commercial logging, or is being cleared to provide land for industrial agriculture, such as oil-palm plantations, sugar-cane fields, and cattle pasture. Tropical deforestation is also caused by flooding during the development of hydroelectric reservoirs, by the cutting of wood to manufacture charcoal, and by the harvesting of fuelwood, especially near towns and cities. Wood is the predominant cooking fuel in many tropical countries, especially for poorer, rural families – for most of the world’s people, the energy crisis involves fuelwood, rather than fossil fuels (see Chapter 14).
Because so many species live in tropical forest, the modern rate of deforestation of this biome is having catastrophic consequences for global biodiversity. This damage will become increasingly important in the future, assuming the present relentless pace of tropical deforestation continues.
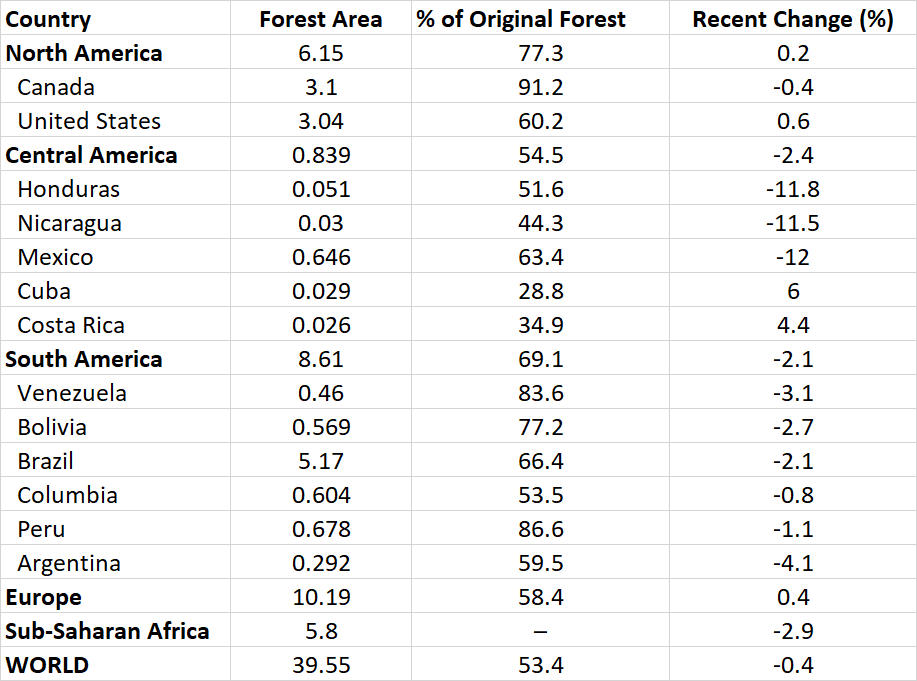
Image 27.2. The greatest modern threats to biodiversity are associated with deforestation in tropical countries. This area in West Kalimantan in Indonesian Borneo was, until recently, covered in old-growth tropical rainforest. The forest was logged to recover its largest trees, which were used to manufacture timber and plywood for export. A secondary harvest was then made of smaller trees for local use, after which the area was converted to agricultural land-use through a practice known as slash-and-burn. At the time the photo was taken, people had just moved into the area and were engaging in subsistence agriculture. Few native species can survive in this ecologically degraded habitat. Source: B. Freedman.

Fortunately, a widespread awareness and concern has developed about this important ecological problem. This has stimulated a great deal of research into the conservation and protection of tropical forests, and governments have started to set aside substantial areas as national parks and other kinds of protected areas. Thousands of sites, comprising hundreds of millions of hectares, have now received some sort of “protection” in tropical countries.
However, the effectiveness of the protected status varies greatly. It depends on factors that influence governmental commitments to conserving forest and other natural ecosystems and to protecting biodiversity more generally. Social stability and related political priorities are important considerations – these are critical to addressing the economic causes of the destruction of tropical ecosystems. Societal factors include poverty, population growth, inequities in the distribution of wealth and land, industrial timber harvesting to earn foreign exchange, and corruption. More directly, political stability and priorities determine whether enough money is available to support a system of protected areas and to find effective means to control the poaching of animals and timber and to prevent other encroachments.
Poaching (illegal harvesting) of endangered wildlife is a terrible problem for species that have economic value on the international black market (see Global Focus 27.1). This can be illustrated by the black rhino (Diceros bicornis) and the elephant (Loxodonta africana) in a game reserve in Zambia, Africa. In the early 1970s, the Luangwa Valley contained about 100-thousand elephants and as many as 12-thousand black rhinos (Leader-Williams et al., 1990). Unfortunately, these relatively large populations quickly collapsed because of poaching, which resulted from the extremely high prices paid for rhino horns and elephant tusks on the black market. Even though Zambian park wardens made courageous efforts under difficult circumstances, it proved impossible to control the poaching. The astronomical value of horn and ivory has spawned a well-organized and profitable chain of illegal poaching, smuggling, and sale.
In spite of these sorts of problems, some tropical countries are developing a real commitment to the protection of their threatened biodiversity. In Central America and the Caribbean, for example, Belize has given protected-area status to 37% of its landbase, while Costa Rica had allocated 27%, and the Dominican Republic 19% (World Bank, 2015). For perspective, we should note that the relative areas of protected land in those Latin American nations are greater than in Canada (8.6%) or the United States (14%), in spite of their comparative poverty (these data are for IUCN categories I–V; see Global Focus 27.2). Such vigorous conservation activities are badly needed in the region: Costa Rica retains only about 35% of its original forest and the Dominican Republic 25%.
In other Latin American countries, conservation efforts have been disrupted by civil war and other political instabilities, and also by indifferent governmental and social priorities. For example, in 2006, the percentage of the national territory with status as IUCN I–V protected areas was only 0.1% in Jamaica, 0.2% in El Salvador, 0.3% in Haiti, 0.6% in Mexico, and 5.5% in Trinidad and Tobago (WRI, 2008).
Global Focus 27.1. Categories of Protected Areas The International Union for the Conservation of Nature (IUCN) and the World Commission on Protected Areas (WCPA) recognize six categories of protected areas. Categories I, II, and III represent particularly strong commitments to maintaining natural ecosystems within the protected area, while the other categories allow some degree of resource management or extraction. The following explains the salient features of the various categories: I. Strict Nature Reserves and Wilderness Areas include ecological, nature, and wilderness reserves. These are managed to preserve their natural condition, although use by scientists for research and monitoring may be allowed. II. National Parks and Equivalent Reserves consist of national, state, and provincial parks, plus areas under Aboriginal or other traditional ownership. These areas are managed primarily to protect ecosystems, although non-consumptive recreation is usually permitted. III. Natural Monuments include geological phenomena and archaeological sites and are intended to protect features of aesthetic or cultural importance. IV. Habitat and Species Management Areas consist of wetlands and wildlife sanctuaries. These are intended to conserve through the protection and management of habitats. Hunting and other consumptive uses may be allowed. V. Protected Landscapes and Seascapes include landscapes, marine areas, scenic rivers, recreational areas, and conservation areas in which the varied interactions of people and nature have produced areas of distinct character. These areas are managed to sustain use by both people and wild species and ecosystems. VI. Managed Resource Protected Areas contain areas of primarily natural ecosystems. These are managed to conserve biodiversity, while also providing sustainable harvests of renewable resources and ecological services.
The world’s greatest expanses of tropical rainforest occur in equatorial Africa, south and southeastern Asia, Central America, western South America, and the basin of the Amazon River. The latter region, known as Amazonia, contains the most extensive rainforest and may support half of the biodiversity of Earth (Mongabay, 2008). This rich tropical region is still extensively covered by primary old-growth rainforest that has been little affected by modern agriculture, lumbering, or other influences of industrial society (although all of Amazonia has supported indigenous cultures for thousands of years).
However, the exploitation and devastation of the Amazonian forest is proceeding rapidly. Great expanses of rainforest are being converted into industrial-scale cattle ranches and soybean farms. In addition, large areas have been deforested by poor farmers who have migrated from heavily populated regions of Amazonian countries in search of “new” agricultural land. Extensive areas of Amazonian forest have also been degraded by hydroelectric developments, lumbering, mining, and timber harvesting to manufacture charcoal as a fuel for the production of iron.
Most of Amazonia lies in northern Brazil. The population in that region has increased enormously in recent decades to several million people, mostly through the migration of landless peasants from other parts of Brazil. This, along with the development of industrial agriculture, has resulted in rapid deforestation in Amazonian Brazil. Between 1970 and 2013, a total of 759-thousand km2 of tropical forest was cleared, equivalent to about 19% of Amazonia in Brazil (Butler, 2015). In total, Brazil has accounted for 80% of the deforestation in Amazonia. The peak of deforestation was in 2004, when about 28-thousand km2 of primary forest was cleared in Brazil. Fortunately, the rate of deforestation has slowed since then, to 6-thousand km2 in 2013. Most of the continuing deforestation is done to develop additional acreage for cattle ranching and soybeans, for which huge export markets have developed in China and Europe.
For various reasons, including pressures exerted by international environmental organizations, the governments of Brazil and other Amazonia countries have committed themselves to conserving their natural heritage, even while vigorously “developing” the economy of the region. Up to 2012, the government of Brazil had designated about 2.0-million km2 of Amazonia as protected areas or Indigenous reserves, while Peru had set aside 365-thousand km2, Venezuela 325k km2, Colombia 309k km2, and Bolivia 220k km2 (Butler, 2015). The protected areas are mostly national parks, while the Indigenous reserves are intended to protect the homelands and cultures of aboriginal peoples. However, as with protected areas everywhere, these ones often suffer from poaching, illegal mining and agricultural settlement, and other prohibited activities that degrade their ecological values, while also threatening Aboriginal cultures and land tenure.
Global Focus 27.2. Trade in Species-at-Risk Some endangered species are valuable for one reason or another. They may be sought by private collectors or by zoos or botanical gardens, which may be willing to pay a large price for a living specimen. Some animal and plant tissues are valuable, which may result in endangered species being killed for their fur, horn, or ivory, or for their finely grained or colourful wood. For example, rhinoceros horn is precious is Yemen for crafting dagger handles, while in eastern Asia tiger bones, the rhizome of ginseng, and bile from the gallbladder of bears are used in traditional medicine. Elephant ivory is valued for carving, and rare furs of cheetah, jaguar, leopard, and tiger are used in expensive clothing.
A global treaty called the Convention on International Trade in Endangered Species (CITES) obliges signatory nations to control the trade in threatened species within their national jurisdiction. CITES was established in 1973 under the United Nations Environment Programme (UNEP). Its key function is to monitor the international trade in endangered species and to control or prevent it as much as possible. For these purposes, species are assigned status as being extinct, endangered, vulnerable, or rare by an allied organization called the World Conservation Union (IUCN). The actual international trade in species is monitored by the “Traffic” network of the IUCN and the World Wildlife Fund (WWF). The global headquarters of CITES, IUCN, and WWF are all in Switzerland.
International trade in 630 species of animals and 301 plants is prohibited by CITES (these are so-called Appendix I species; CITES, 2015). In addition, the trade in 4,827 animals and 29,592 plants (Appendix II) requires a CITES permit and is monitored by the World Conservation Monitoring Centre (WCMC) of Cambridge, UK. WCMC also publishes a series of “red books” that summarize the status and commerce of about 8,300 plant and 7,200 animal species. Canadian species listed by CITES include 49 species of mammals, 57 birds, 4 reptiles, 9 fish, and 85 plants (the latter are mostly native orchids; Environment Canada, 2015). However, the importing of a much larger number of non-Canadian species listed by CITES is also monitored and regulated by Environment Canada.
One of the responsibilities of Canada under the CITES treaty is to report on its international trade in species that fall under the purview of WCMC. In 2013, for example, the Government of Canada issued 1,097 permits to export CITES species or their parts (CITES, 2015). Many of the permits are for species that are listed by CITES, but are not native to Canada but may be bred here, such as parrots, cacti, and orchids. Table 1 shows a selection of native Canadian species for which CITES export permits were given in 2013.
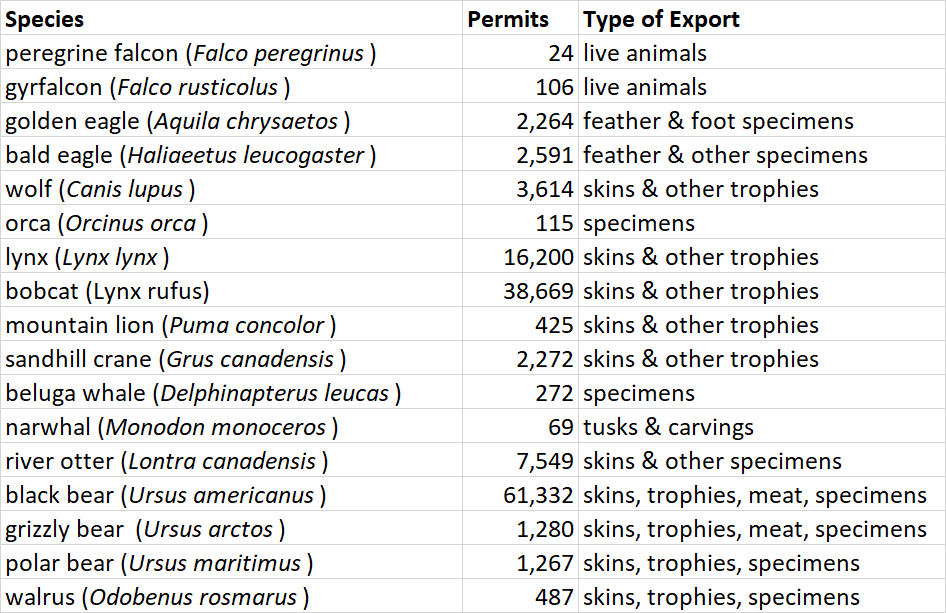
Of course, these data refer only to the legal trade of species listed by CITES. There is also an illegal trade in Canada, particularly of bear gallbladders, caribou antlers, and certain furs. There is also an illegal trade in some living animals and plants such as certain orchids, and gyrfalcons and peregrine falcons that are valuable in the Middle East for the sport of falconry. Most of the illegal trade involves animals and plants that were hunted or collected by poachers. In addition, there are large illegal imports of banned products into Canada, such as rare parrots, reptiles, and fish for the pet trade. There is also a burgeoning illicit trade of animal parts used in traditional medicine, particularly to service a large market in traditional Chinese medicine.
The illegal trade in rare and endangered species is responsible for an enormous international economy of as much as several billion dollars per year (reputedly second only to the illegal drug trade). This is the reason why this kind of illegal commerce is flourishing in so many countries, including Canada. To some degree, governments can deal with the problem by more rigidly enforcing their laws governing the illegal trade and by imposing severe penalties on convicted offenders. Ultimately, however, the illicit commerce is driven by a wealthy and enthusiastic marketplace. Obviously, for the sake of the endangered biodiversity, it is crucial that the demand be curtailed as soon as possible. Ultimately, people’s attitudes must be changed, and severe penalties must be imposed for the illegal possession of species or body parts banned by CITES.
Species Declines
Numerous species of certain groups of organisms have been suffering intense and widespread declines in their populations, with many of them becoming endangered and even extinct. These include large carnivores, reptiles, amphibians, predatory birds, and migratory songbirds. We will examine the problem of species declines using the example of North American songbirds.
Within the past two decades or so, ecologists and birders have been reporting alarming declines in the populations of many species of so-called neotropical migrants (these are birds that spend most of the year in tropical habitats but migrate to higher-latitude regions to breed). Most of the declining species breed in mature temperate and boreal forest. Although the reasons for the songbird declines are not totally understood, the most important factors are probably the following:
- extensive deforestation in their tropical wintering range
- disturbance of mature-forest habitat in the northern breeding range
- fragmentation of the breeding habitat into “islands” that are too small to sustain populations over the long term, and that are easily penetrated by forest-edge predators and nest parasites (such as cowbirds – to be discussed later)
- loss of critical habitats for staging and migration
- effects of pesticides and other toxic chemicals
- possibly also new introduced diseases, such as the West Nile virus
Bachman’s warbler (Vermivora bachmanii) appears to have become extinct because of the loss of its tropical wintering habitat. This songbird used to breed in mature hardwood forest in the southeastern United States. Although suitable habitat remains in that region, this warbler has not been seen since the mid-1950s and is undoubtedly extinct. This loss was probably caused by the clearing of its critical wintering habitat, believed to have been humid tropical forest in Cuba that was converted into sugar-cane plantations.
Much of the evidence suggesting that populations of many other neotropical migrants are declining is anecdotal – skilled birders are not seeing as many individuals of many species as they once did, even in places where the local habitat has not changed much. Unfortunately, only a few studies have closely monitored bird populations for many years in mature forest habitat. One of the best long-term studies is for a tract of forest in West Virginia, where the breeding birds, particularly the migrants, declined substantially over a 37-year period. From 1947 to 1953, 25-28 species bred at that site, of which 14-16 were neotropical migrants. This decreased to only 15 species and 8 migrants breeding over the period of 1973-1983 (Terborgh, 1989). During that same period, the total abundance of birds decreased by 16%, and that of neotropical migrants by 37%. In another important census of forest birds, made at Hubbard Brook, New Hampshire, 70% of the breeding species declined between 1969 and 1984 (Holmes et al., 1986).
An important data set has been compiled by the Canadian Wildlife Service, based on information from a large number of breeding bird surveys, which are made annually at many locations using a common methodology. Because so many widely spaced areas contribute to the database, it provides an indication of synoptic trends in the abundance of birds. The analysis in Figure 27.2 shows that the abundance of neotropical migrants breeding in Canada has declined markedly, while that of species that spend the winter in North America has not decreased to the same degree.
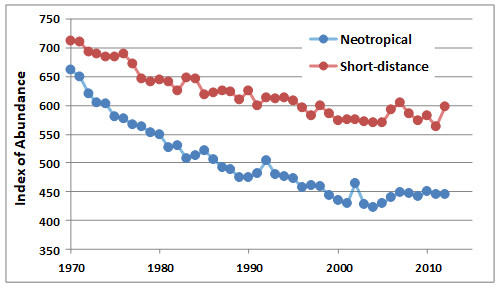
The causes of the declines of migratory landbirds include the reduction of their breeding habitat due to timber harvesting and conversion into agricultural and urbanized areas. The amount of high-quality habitat has declined, while much of the remainder has been fragmented into small islands of natural forest. This change is important because birds have less success when breeding in small fragments of habitat. In part, this is because their nests are more vulnerable to predators such as crows, jays, magpies, skunks, and foxes.
Many migratory species have also been affected by nest parasitism by the brown-headed cowbird (Molothrus ater), which lays its eggs in the nests of other species. The foster parents raise the voracious cowbird chick, while their own young are neglected and usually die. The cowbird has greatly expanded its range and abundance in North America, mostly because humans have provided it with suitable habitat by disturbing the formerly extensive forest. Cowbirds feed in open areas and are particularly efficient at parasitizing nests near forest edges.
Many bird species in the northern and eastern parts of the modern range of the cowbird are extremely vulnerable to nest parasitism (Freedman, 1995). They have only recently come in contact with this parasite and have not evolved an effective defense. For example, Kirtland’s warbler (Dendroica kirtlandii), an endangered species, may suffer a parasitism rate of 70%, and each incidence leads to reproductive failure. A study in Illinois found that two-thirds of 75 nests of various host species were parasitized by cowbirds, including 76% of 49 nests of neotropical migrants. The rate of nest parasitism of white-crowned sparrows (Zonotrichia leucophrys) in California increased from 5% in 1975 to 40-50% in 1990-1991, much more than the 20% rate that the population could face without declining.
The cowbird problem is a dilemma. This is because the only obvious way to help the threatened birds is to kill large numbers of cowbirds, itself a native species. Although distasteful, that action is required if people wish to deal with the severe damage that this parasite is inflicting on other birds, as an indirect consequence of anthropogenic changes to its habitat.
Back from the Brink
Fortunately, dismal stories about extinctions and other grievous losses do not make up all the news about biodiversity. There are also some uplifting successes of conservation. These involve species that were taken perilously close to the brink of extinction, but have since recovered because they were given effective protection. In some cases, the recoveries have been vigorous enough that the species are no longer in imminent danger. Although these success stories are a distinct minority (the number of endangered species is increasing much more rapidly), they are nevertheless instructive. They illustrate that positive actions can yield great benefits, both for the species in question and for the people that may now be able to exploit them as a potentially renewable resource. (Historical data in this section are from Freedman, 1995.)
The Northern Fur Seal
The northern fur seal (Callorhinus ursinus) lives in the northern Pacific Ocean. It was relentlessly exploited for its fur, and by 1920 had been reduced from a population of several million to only about 130-thousand. Because this fur seal was believed to be in danger of extinction, an international treaty was signed to strictly regulate its harvest. Its population responded vigorously to the conservation measures and rebounded to almost 1-million individuals, abundant enough to again support a commercial hunt for its fur, leather, and oil. However, its numbers have declined again and now there is only a subsistence hunt, equivalent to about one-thousand animals per year since 2000 (NMFS; 2007). The northern fur seal has been suffering considerable non-hunting mortality from entanglement in fishing gear, over-fishing of its food, poaching, and oil spills.
Some other seals were also hunted excessively, but then rebounded in abundance after the exploitation was stopped, or at least sensibly regulated. Two Canadian examples are the harp seal (Phoca groenlandica) of the north Atlantic Ocean, which now numbers about 7-million animals (Chapter 14), and the grey seal (Halichoerus grypus) of temperate Atlantic waters. The grey seal numbered only about 5-thousand animals as recently as the mid-1960s and was considered endangered. Since then, however, this seal has had remarkable population growth and now numbers more than 0.5-million animals.
The Whaling Industry
Many populations of large whales were severely depleted during several centuries of unregulated hunting (Chapter 14). Following protection, some populations have substantially recovered. The best example of this is the grey whale (Eschrichtius robustus) of the Pacific coast of North America, which was protected in the 1930s when its population numbered as few as one-thousand animals. It now numbers about 19-thousand, roughly comparable to its pre-exploitation abundance (IWC, 2015). Although the grey whale of the eastern Pacific is no longer endangered, it was extirpated in the eastern Atlantic several centuries ago, and a tiny stock in the western Pacific is critically endangered.
Other large whales were also heavily depleted by commercial hunting. With few exceptions, they have been protected from exploitation since an international moratorium on whaling in 1986. Their populations are slowly recovering, although not yet to the degree achieved by the grey whale. The sperm whale (Physeter catodon), for example, had a global pre-whaling abundance of about 2-million, but now numbers fewer than 1 million (IWC, 2015). Similarly, the finback whale (Balaenoptera physalus) initially numbered about 200-thousand, but now numbers 50-thousand. The blue whale (B. musculus), initially numbering 200-thousand, now numbers only 6.5-thousand. The humpback whale (Megaptera novaeangliae) was about 120-thousand and is now 89-thousand. These species will continue to recover their abundances as long as they remain protected from commercial hunting. There is intense pressure, however, for the moratorium to end for the most abundant species, particularly the minke whale (Balaenoptera acutorostrata), which numbers about 600-thousand.
Image 27.3. Populations of humpback whales (Megaptera novaeangliae) were heavily exploited worldwide by commercial whaling. However, this species is now protected and its numbers are increasing. Humpback whales, such as this animal off Brier Island, Nova Scotia, spend much of the summer feeding on small fish in northern latitudes. Source: B. Freedman.

Several other species of whales remain badly depleted and are recovering extremely slowly. One of these is the population of right whales (Balaena glacialis) of the western Atlantic, which numbers only about 500 individuals. Another is the right whale of the eastern Pacific Ocean, with only a hundred or so animals. Yet another is the bowhead whale (Balaena mysticetus) of the Arctic, with a population of about 14-thousand. Bowhead whales are still subjected to an Aboriginal hunt in northern coastal Alaska (no more than 67 can be struck per year; several are also taken off Baffin Island in Canada and Greenland). The most important causes of mortality of Atlantic right whales appear to be collisions with ships and entanglement in fishing nets.
The American Bison
Before the American bison (or buffalo; Bison bison) was subjected to an intensive commercial hunt, its population was about 60 million. At that time, bison was the most abundant large wild animal in North America, ranging over most of the continent.
The eastern subspecies (B. b. pennsylvanicus) was an animal of forests and glades that ranged over much of the eastern United States. It was hunted to extinction by the mid-1800s. The plains bison (B. b. bison) ranged throughout the prairies and was by far the most populous subspecies. They migrated in enormous herds – one was described as being 80 km long and 40 km wide, another as 320 km long, and another as moving over a 160 km front! The plains bison were subjected to an intensive market hunt during the nineteenth century and were nearly exterminated. Apart from the money that was made by selling meat and hides, the eradication of these bison was encouraged by governments, especially in the U.S., likely for two reasons. First, the development of prairie agriculture was being disrupted by the bison herds, especially during their mass migrations. Second, because the bison were critical to the subsistence economy of the Plains Indians, extermination of these abundant animals made it easier to displace the Aboriginal tribes in favour of European colonists.
The most famous buffalo hunter was William F. Cody, or “Buffalo Bill,” who was contracted in 1869 to provide meat for workers constructing the Union Pacific Railroad through the U.S. prairie. Cody reportedly killed 250 bison in a single day and more than four-thousand during an 18-month period. Once the railways were built, tourist excursions were organized during which a train would stop near a herd of migrating buffalo, which allowed passengers to shoot them in a leisurely fashion through windows of the coaches. Some of the tongues (a delicacy) would be cut from the dead animals, and perhaps some hides, but otherwise the carcasses were left to rot. Such actions were a wanton destruction, but the worst damage was caused by market hunts that were made feasible by the new railroad, because it allowed the meat to be quickly shipped to urban consumers. Between 1871 and 1875, market hunters killed about 2.5 million bison per year. In addition, the Plains Indians had acquired rifles and horses by this time and were also able to hunt bison much more effectively than before.
The unregulated hunting of the plains bison was unsustainable, and the species declined precipitously. By 1889 there were fewer than one-thousand bison left in the United States, and only small herds survived in the Canadian prairies. Almost too late, a few closely guarded preserves were established, and some wild animals were captured for breeding programs. These and later actions have allowed the numbers of plains bison to increase to their present abundance of more than 50-thousand animals. Of course, almost all of their original habitat is gone, having been converted into agriculture, so this animal will never recover its former abundance. However, the plains bison is no longer endangered.
The wood bison (B. b. athabascae) of the southwestern boreal forest is a third subspecies. When this animal became endangered by over-hunting, its only remaining wild population was protected in and around Wood Buffalo National Park in northern Alberta and the adjacent Northwest Territories. Unfortunately, the genetic integrity and health of this population has been degraded by interbreeding with plains bison, which were introduced to the area by misguided wildlife managers in the late 1920s. However, a previously unknown population of wood bison was discovered in 1960 in a remote area of Wood Buffalo National Park, which appears not to have suffered from interbreeding with the plains subspecies. Some of these “pure” wood bison were used to establish another isolated population, northwest of Great Slave Lake. Regrettably, many of the wild-ranging wood bison have been exposed to introduced diseases of cattle, most notably tuberculosis and brucellosis. These, along with predation by people and wolves, have taken their toll, and the long-term viability of their population is cause for concern.
Remarkably, agricultural interests within the federal government have proposed to exterminate virtually all of the bison in the vicinity of Wood Buffalo National Park, except for the “pure” wood bison occurring in isolated populations known to be free of bovine diseases. That slaughter would be intended to prevent the spread of brucellosis and tuberculosis from bison to cattle herds that are spreading northward in Alberta. A secondary reason for the proposed slaughter is to protect the genetic integrity of the wood bison subspecies, because hybrid wood/plains animals would be targeted for extermination. This would leave the more isolated, non-diseased, pure wood bison to repopulate the cleared habitat. This proposal is highly controversial and, even if permitted, would probably not be successful. An enormous effort would be required to find each and every hybrid bison in the target area, which is an immense wilderness of boreal forest and muskeg.
Some Other Recoveries
The sea otter (Enhydra lutris) lives on the west coast of North America. This marine mammal was subjected to a devastating 18th- and 19th-century hunt for its dense and lustrous fur. In fact, it had been thought to be extinct, until small residual populations were discovered in the 1930s. The sea otter has now recovered its abundance over much of the west coast. This resurgence was aided by re-introductions into areas from which it had been extirpated, such as the Pacific coast of Vancouver Island. Sea otters now number more than 100-thousand individuals, but the Canadian population is still small enough to be designated as a threatened species.

The pronghorn antelope (Antilocapra americana) of the western plains was severely over-hunted during the nineteenth century, and its population was reduced to about 20-thousand individuals. Fortunately, strong conservation measures were implemented, and this species now numbers more than 500-thousand, and it again sustains a sport hunt.
The trumpeter swan (Cygnus buccinator) used to breed extensively in western North America, but its populations were devastated by hunting for its meat and skin, with perhaps fewer than 100 surviving. However, this swan is now protected and has recovered in abundance, now numbering more than 24-thousand individuals.
The wild turkey (Meleagris gallopavo) was widely extirpated from its natural range by hunting and habitat loss (of course, domestic varieties are abundant in agriculture). Because of conservation measures and re-introductions to areas from which the species had disappeared, populations of wild turkeys have recovered substantially, for example in southern Ontario and Quebec. Many stocks of this large gamebird can again sustain a sport hunt.
The wood duck (Aix sponsa) was over-hunted for its beautiful feathers and as food. It also suffered from losses of habitat due to lumbering and wetland drainage. The recovery of the wood duck has been aided by the widespread provision of nest boxes in wetlands used by this cavity-nesting species. Nest-box programs also benefit several other relatively uncommon cavity-nesting ducks, particularly the hooded merganser (Lophodytes cucullatus) and common goldeneye (Bucephala clangula). An unrelated program of providing terrestrial nest-boxes has helped to increase the abundance of eastern and western bluebirds (Sialia sialis and S. mexicana), which had been declining because of habitat loss.
The American beaver (Castor canadensis) was one of the most sought-after species in the fur trade, a commercial activity that stimulated much of the early exploration of Canada and the United States. Beavers were over-harvested almost everywhere, which caused the species to be extirpated from most of its natural range. However, conservation measures and decreased demand for its fur have allowed the beaver to recover its populations over most of its range where the habitat is still suitable. In fact, they are now considered to be a “pest” in some recolonized habitats because of the flooding they cause.
The whooping crane (Grus americana) is, it is hoped, an incipient success story of conservation. The whooping crane was never very abundant (likely around 1,500 individuals), even before its populations were devastated by the combined effects of hunting, loss of its breeding habitat of prairie wetlands to agriculture, deterioration of its wintering habitat along the Gulf of Mexico, and egg and specimen collecting. These stressors drove the wild population down to a perilously small level of only 15 individuals (in 1941). Fortunately, since then, the whooping crane has been vigorously protected from hunting, while its major breeding habitat in Wood Buffalo National Park and its wintering habitat in coastal Texas have been conserved. These measures, along with a program of captive breeding and release, have allowed the population of whooping cranes to increase to more than 600 animals (in 2011; almost one-third are in captivity, 279 in the Wood Buffalo population, and the rest in newly established breeding populations in Wisconsin and Florida). There is cautious optimism for the survival of this species, although it is still endangered.
Canadian Biodiversity at Risk
The conservation status of species in Canada is assessed by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). COSEWIC is a consultative body with expert representatives from governments (federal, provincial, territorial, and Aboriginal), universities, and non-governmental organizations. It makes recommendations to the federal, provincial, and territorial governments, whose responsibility it is to actually designate conservation status.
Once a species is listed as endangered or threatened in Canada, a parallel body known as RENEW (REcovery of Nationally Endangered Wildlife) is mandated to prepare a plan that would ensure the recovery of its population to a safer level. However, because of a lack of funding, as of 2014 only about 40 recovery plans had been completed, although many others were in various stages of development.
COSEWIC recognizes five categories of risk, each of which has a specific meaning in terms of imminent threats to the future survival of species (COSEWIC, 2015).
- Extinct refers to any species of wild life that was formerly indigenous to Canada but no longer exists anywhere in the world. Canadian examples of extinct species are the great auk (Pinguinus impennis), passenger pigeon (Ectopistes migratorius), Labrador duck (Camptorhynchus labradorium), sea mink (Mustela macrodon), deepwater cisco (Coregonus johannae), longjaw cisco (Coregonus alpenae), and eelgrass limpet (Lottia alveus). Extinct subspecies are the Queen Charlotte caribou (Rangifer tarandus dawsoni), blue pike (Stizostedion vitreum glaucum), and Banff longnose dace (Rhinichthys cataractae smithi). As of 2014, 15 Canadian taxa (species, subspecies, or distinct populations were extinct.
- Extirpated refers to any species or subspecies that was formerly indigenous to Canada but now only survives in the wild or elsewhere, usually in the neighbouring United States. Examples include the black-footed ferret (Mustela nigripes), Atlantic grey whale (Eschrichtius robustus), Northwest Atlantic walrus (Odobenus rosmarus), greater prairie chicken (Tympanuchus cupido), pygmy short-horned lizard (Phrynosoma douglassi), paddlefish (Polyodon spathula), and blue-eyed mary (Collinsia verna). As of 2014, 23 taxa were extirpated in Canada.
- Endangered refers to indigenous species that are faced with imminent extinction or extirpation throughout all or a significant portion of their Canadian range. As of 2014, 312 taxa were considered to be endangered. Examples include the Vancouver Island marmot (Marmota vancouverensis), bowhead whale (Balaena mysticetus), right whale (Balaena glacialis), whooping crane (Grus americana), Eskimo curlew (Numenius borealis), burrowing owl (Speotyto cunicularia), piping plover (Charadrius melodus), Blanchard’s cricket frog (Acris crepitans blanchardi), blue racer snake (Coluber constrictor foxii), eastern prickly pear cactus (Opuntia humifusa), small white ladyslipper (Cypripedium candidum), thread-leaved sundew (Drosera filiformis), and seaside centipede lichen (Heterodermia sitchensis)
- Threatened refers to any indigenous taxon that is likely to become endangered unless factors affecting its vulnerability are reversed. As of 2014, 167 taxa were considered threatened in Canada. Examples include the wood bison (Bison bison athabascae), sea otter (Enhydra lutris), Pacific humpback whale (Megaptera novaeangliae), marbled murrelet (Brachyramphus marmoratus), massasauga rattlesnake (Sistrurus catenatus), and American chestnut (Castanea dentata).
- Special concern refers to any indigenous species that is not currently threatened but is at risk of becoming so because of small or declining numbers, occurrence at the fringe of its range or in restricted areas, habitat fragmentation, or some other reason. As of 2014, 204 taxa or populations were considered to be of special concern. Examples include the grizzly bear (Ursus arctos), polar bear (Thalarctos maritimus), blacktail prairie dog (Cynomys ludovicianus), long-billed curlew (Numenius americanus), ivory gull (Pagophila eburnea), spotted turtle (Clemmys guttata), and eastern prairie fringed orchid (Platanthera leucophaea).
It must be recognized that the designation of species at risk is a continuing and always incomplete process. For instance, because the conservation status of only a few species of invertebrates has been investigated, endangered species in this group are enormously under-represented in the COSEWIC list. Unfortunately, more rapid progress is constrained by a shortage of funding for research and monitoring of endangered species, and by a lack of specialists with the necessary taxonomic and ecological skills and knowledge.
In addition to the work of COSEWIC, an intergovernmental group of federal, provincial, and territorial scientists is working to develop periodic science assessments of the status of Canadian biodiversity. Their most recent evaluation provides an excellent appraisal of the prospects and information needs of a wide swath of Canadian species, and is a valuable source of information (CESCC, 2014).
Of course, it is not sufficient merely to designate species as being at risk of extirpation or extinction. If their status is to be improved, the species and their habitats must also be protected. Remarkably, governments in Canada have not yet enacted effective legislation to protect endangered species and their habitat. However, this situation is starting to change. In 2002, the Government of Canada passed a Species at Risk Act, which provides some protection for species occurring on federal lands and otherwise within federal jurisdiction.
However, the federal legislation has little direct influence on the status of the many species-at-risk that are living on provincial, territorial, Aboriginal, or private land. Most importantly, the Act does not fully address the protection of habitat of endangered species off federal land. To some degree, this deficiency is covered by legislation that has been enacted by provinces and territories. However, their legislations are also not very effective, because they too do not specifically protect the habitat of species at risk, especially on private land. Such a piecemeal approach results in uneven levels of protection for species-at-risk, which is unacceptable from the conservation viewpoint.
The lack of effective protection of species-at-risk in Canada is raising controversy. Governments feel the need to demonstrate that they are making rapid progress toward sustainability, an important component of which involves the protection of native species and their habitats. Unfortunately, the progress to date has been lacking and is not yet effective in protecting our endangered biodiversity. Hopefully, the lobbying efforts of Canadian non-governmental organizations will result in appropriate changes to the currently weak legislation of all levels of government. Key national organizations in this regard are Nature Canada, Canadian Parks and Wilderness Society (CPAWS), Ecojustice, and World Wildlife Fund-Canada (WWF).
Some of the natural ecosystems of Canada now exist only as small remnants of their former extent. Because of this, they are as endangered as the species they support. The most endangered of our natural ecosystems are (see also Chapter 8):
- the tall-grass prairie of southwestern Ontario and southeastern Manitoba
- Carolinian forest of southern Ontario
- dry coastal Douglas fir and Garry oak forest types of southwestern British Columbia
- semi-desert of southeastern British Columbia
- various kinds of old-growth forest in all parts of forested Canada, but particularly in the east
- and many kinds of freshwater wetlands
Some of these ecosystems, particularly the tall-grass prairie and Carolinian forest, are also rich in endangered species. It is imperative that the remaining areas of these endangered ecosystems become preserved in parks and other kinds of protected areas.
Protected Areas
Protected areas are parks, ecological reserves, and other tracts of land or water that have been set aside from intensive development to conserve their natural values. The intent is usually to protect representative examples of widespread communities or ecoscapes, threatened ecosystems, or the habitat of endangered species. However, many protected areas also support human activities that do not severely threaten the ecological values that are being conserved. Such activities may include ecotourism, other kinds of non-consumptive outdoor recreation (such as skiing and golf), spiritual activities, education, scientific research, and sometimes even exploitative activities such as hunting, fishing, trapping, or timber harvesting (see Environmental Issues 27.1).
It is important to understand that protected areas should not be regarded as the only, or even as the most important way to conserve endangered species and ecosystems. To the degree possible, native species and other natural values should be accommodated in all areas that people are using for economic purposes, such as for agriculture, forestry, fishing, or mining. The role of protected areas is to ensure that species and ecosystems that are at risk in those “working” areas still have suitable refuges where they can maintain themselves.
Ideally, a national system of protected areas in Canada would involve lands and waters controlled by federal, provincial, and territorial governments, Aboriginal groups, and private interests. A perfect system would be designed to sustain all native species and natural ecosystems over the long term, including terrestrial, freshwater, and marine systems. To ensure that all elements of native biodiversity are adequately represented within a system of protected areas, all species and ecosystem types in the country or province must be identified, their abundance or extent determined, and their critical stressors understood. This information would allow all aspects of natural-ecological heritage to be accommodated within a comprehensive system plan for a network of protected areas.
Of course, these are ideal criteria, and no country has yet designed and implemented a comprehensive network of protected areas that sustains all native species and natural ecosystems. Moreover, most existing protected areas are relatively small and are threatened by stressors originating within their boundaries or by degrading influences from the surrounding area. Because of these and other problems, it is doubtful whether many of the smaller protected areas will be able to sustain their present ecological values over the longer term. This will be especially true if a major environmental change occurs, such as global warming or a catastrophic disturbance (such as a devastating wildfire or disease epidemic).
The International Union for the Conservation of Nature (IUCN) recognizes six categories of protected areas (see Global Focus 27.2). In 2011, there were about 161-thousand terrestrial protected areas in the world, representing as much as 15% of the global land area (Protected Planet, 2015). There are many fewer marine protected areas, about 7-thousand and covering only 1% of the oceanic surface. Each year, however, additional protected areas are added to the tally, including in the marine realm, for which such initiatives are recent compared with the terrestrial realm.
Protected Areas in Canada
National parks, provincial parks, and similar places are the largest and most important protected areas in Canada. A summary of areas protected in Canada is provided in Figures 27.3 and 27.4. Note that the nationally protected area of about 10% is smaller than recommended by many conservation scientists, whose estimates range from 15-40% of the landmass.
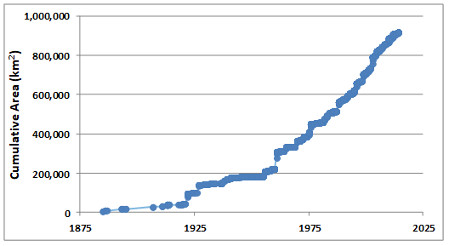
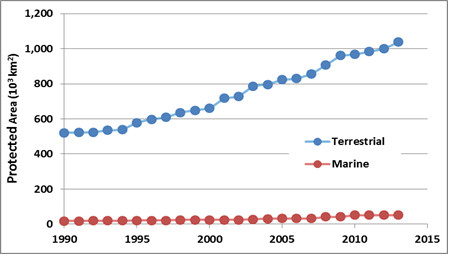
Figure 27.4. Protected Areas in the Regions of Canada. Source: Environment Canada (2015b).
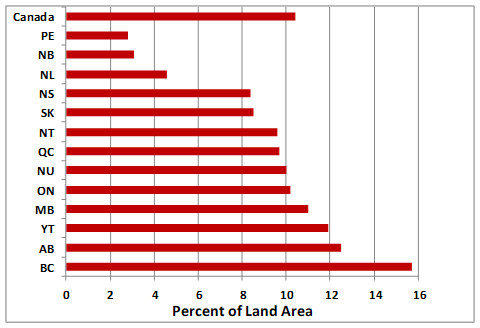
In addition to their primary goal of conserving biodiversity, most parks serve additional purposes, including the support of economically important outdoor recreation and tourism. To some degree, the use of parks for these purposes is a challenge to their ability to function as ecological reserves. For example, strictly interpreted, the ecological values of national parks are not compatible with consumptive uses of their natural resources (such as sport fishing) or with the development of infrastructure to support recreation and tourism (such as campgrounds, golf courses, hotels, roads, ski facilities, and even interpretation facilities).
The ecological-reserve function of many protected areas is also threatened by land-use and management activities that are occurring in their surrounding area. Usually, the most important of the “external” stressors are associated with agriculture, forestry, mining, tourism, or hydroelectric development. In fact, all of the national parks in more southern regions of Canada are significantly threatened in this way. We can illustrate this problem with several well-known examples.
- Point Pelee National Park is a small, 15.5 km2 park in southwestern Ontario. It contains some of the most important remnants of natural habitat left in the Carolinian zone (Chapter 8), most of which has been converted into agriculture or urbanized land-uses. Consequently, Point Pelee supports populations of many endangered species and ecological communities. However, this small park is used intensively for outdoor recreation, including birding, boating, hiking, and picnicking on its beaches. To support these culturally and economically important activities, much of the limited area of the park is maintained as paved roads, pathways, parking lots, campgrounds, information centres, lawns, and other land-uses that do not enhance the protection of ecological values. Moreover, the area next to the national park is almost entirely converted into agricultural lands, such as onion fields established on drained marshes, or into cottage and motel developments that support tourism. These land-uses have isolated the relatively natural ecosystems of Point Pelee, to the degree that it is an ecological “island” that is surrounded by incompatible uses of the landscape. For these and other reasons, the park is losing some of the natural features it is trying to protect. For example, it has lost 10 of its original 21 species of reptiles, and 6 of 11 amphibians. Some of its habitats are being badly degraded by invasions of alien plants (such as garlic mustard, Alliaria petiolata), which crowd out native species. In fact, 37% of the vascular plants in the park are non-native.
- Fundy National Park in New Brunswick is a similar case, although its ecological values are not as severely threatened as those of Point Pelee. Fundy has an area of 206 km2, but park ecologists believe that this is not large enough to sustain viable populations of certain wide-ranging species, such as black bear, pine marten, and pileated woodpecker, or certain natural ecosystems such as old-growth forest. To some degree, these and other natural values are being compromised by the development of tourism facilities within the park, including campgrounds, a golf course, a swimming pool, interpretive facilities, and extensive lawns and roads. Also important are industrial activities in the area surrounding the park, where forestry interests are extensively converting the natural forest into faster-growing conifer plantations
- Banff National Park in southwestern Alberta was the first national park to be established in Canada, in 1885. The original intent was to protect extremely scenic viewscapes and hot springs and to develop the area in support of the economic benefits of tourism. It was not until several decades later that the philosophical underpinning of national parks shifted toward the protection of natural values. In any event, the early development of Banff featured the enthusiastic construction of large hotels, golf courses, skiing facilities, major highways, a transcontinental railroad, several villages, and other structures. This pattern continues today with much ongoing construction, coupled with rapid development of the area east of the park for tourism, residential neighbourhoods, forestry, and other uses. These facilities severely threaten the long-term viability of the natural values of Banff. This economic development is engendering intense controversy and has been the subject of a commission of enquiry (see Environmental Issues 27.1).
Provincial and territorial governments also have a responsibility to protect natural values within their jurisdiction. These governments have designated many ecological reserves and wilderness areas, supplemented by natural areas that are protected in provincial parks and conservation areas, which are also well-used for recreation. Some municipalities also have natural-area parks that provide habitat for native species. An outstanding example is the city of Windsor, Ontario, which is protecting important remnants of tall-grass prairie and Carolinian forest, and their many species-at-risk.
Some environmental non-governmental organizations (ENGOs) are also active in the protection of natural areas. At the national level, the Nature Conservancy of Canada is the ENGO that most actively protects land to conserve its biodiversity, usually by purchasing or accepting donations of private property or land-use rights (see Canadian Focus 27.2). Ducks Unlimited Canada plays a similar role, but with a focus on wetland habitat. At provincial and more local levels, many private land trusts are also protecting natural areas.
Additional national ENGOs also play important roles in protecting the biodiversity of Canada. Prime examples include the Canadian Parks and Wilderness Society, the Canadian Wildlife Federation, Nature Canada, the Sierra Club, and the World Wildlife Fund (Canada). However, these organizations mostly do this work through advocacy – they lobby governments and the private sector to pursue more effective biodiversity agendas. They also engage in public campaigns and conduct research toward those ends. WWF-Canada, for example, was the prime mover behind the Endangered Spaces Campaign, which was effective in convincing governments to preserve representative areas of natural ecosystems within protected areas. The Canadian Council of Ecological Areas is an association of conservation experts in government, ENGOs, and universities, who are working toward a strategic plan for a national system of protected areas.
In spite of the diverse conservation-related activities of governments and private organizations, the existing network of protected areas is highly incomplete. There are four reasons for making this statement:
- the full breadth of Canada’s natural heritage is not yet represented in protected areas
- there are many species at risk in Canada, many of which will have to be protected in ecological reserves that do not yet exist
- most of the existing protected areas are too small to protect their ecological values over the long term
- where species- and communities-at-risk occur in “working” landscapes outside of protected areas, their special needs for critical habitats are often not being met
The third is important because small areas cannot usually sustain viable populations of some species of wildlife over the long term, even if they are protected. Small reserves also cannot sustain the ecological conditions that are required for certain communities to persist, especially old-growth forest. In such cases, a protected area must be managed within the context of its surrounding landscape as a single, integrated ecosystem. Management activities in such greater protected areas should be designed to ensure the long-term viability of populations of species at risk, as well as natural communities at risk.
Environmental Issues 27.1. Ecological Integrity in the Bow Valley In 1885, Banff was the first national park to be proclaimed in Canada. Banff is also the most famous of our national parks, because of its spectacular scenery, easily viewed large animals, and superb infrastructure supporting world-class tourism and outdoor recreation. These values attract visitors from across Canada and many other countries.
Banff National Park covers a large area (6,640 km2) and thus plays an important role in protecting the natural ecological values of its region. This is enhanced by the fact that Banff is bordered by several other protected areas, namely Jasper, Yoho, and Kootenay National Parks and Peter Lougheed Provincial Park, which collectively comprise an area of 26-thousand km2.
Most of the native species and natural ecosystems of Banff are well protected within its boundaries and in surrounding lands. Some others, however, are not. These natural values are threatened by a variety of stressors, some of which exert their influence within the park while others make themselves felt outside its boundaries.
Banff hosts more than 3 million tourists each year, generating more than $6 billion in economic activity. To service its many visitors, the park contains hotels, lodges, and campgrounds. To provide the tourists with interesting things to do, and to generate revenue and local employment, the park contains ski hills with associated lifts and lodges, golf courses, an extensive network of roads and trails, interpretation facilities, and two full-service settlements with more than 8-thousand residents – the villages of Banff and Lake Louise. In addition, the Canadian Pacific Railway passes through Banff, as does the Trans-Canada Highway. These various facilities are developed especially intensely in the so-called Banff-Bow Valley corridor, a region that encompasses the major transportation routes through the park as well as the main tourist areas.
The tourism- and transportation-related infrastructure in the Banff-Bow Valley corridor provides support for big-business tourism and the national system of ground transportation. However, these facilities are stressors to the natural values of the park. In fact, local populations of grizzly bear, timber wolf, and other wide-ranging species are at risk in the greater Banff region, mostly because they suffer unsustainably high death rates. The mortality is a result of collisions with vehicles on the highways and railroad, hunting outside of but close to the park, and the killing of “problem” bears that become habituated to people near campgrounds and frequently used trails. One study of grizzly bears found that 90% of their deaths in Banff occurred within 0.5 km of human infrastructure, and only 2 of 73 deaths were due to natural causes. In addition, the wilderness values of extensive areas have become degraded by visual and noise pollution associated with traffic, highways, railroads, ski lifts, buildings, and large numbers of people.
The various environmental challenges to the ecological integrity of Banff National Park are an increasingly serious problem. A Task Force of five independent experts, appointed by Parks Canada, studied these challenges (Page et al., 1996). The Task Force was given three objectives:
- to develop a vision for the region that would integrate ecological, social, and economic values
- to undertake an analysis of existing information, and to provide direction for monitoring programs
- to recommend changes that would allow the Banff-Bow Valley region to be used for sustainable tourism and recreation industries, while also protecting its heritage of ecological values
The Task Force reviewed a wealth of existing information, commissioned original research, and engaged in public consultations. Its final report concluded that intensive economic development in the Banff–Bow Valley region was quickly approaching an unsustainable level, and this was threatening the ecological integrity of the national park. The Task Force made numerous recommendations for specific actions and policies that would help to deal with the intensifying crisis. It strongly advised that the pace and intensity of development be strictly controlled, and in some cases reversed. In essence, the Task Force reasonably concluded that Banff National Park can be an effective protected area only if its use by people is kept within sustainable bounds.
The Task Force report was favourably received by the then-minister responsible for Parks Canada, who declared that all of its major recommendations would be followed. If this was to happen, however, Parks Canada had to take firm action against powerful economic interests that are determined to increase the amount of recreation and transportation infrastructure in the Banff-Bow Valley corridor. Since then, however, subsequent ministers and senior administrators in Parks Canada have not had the fortitude to resist many of the compelling calls for additional “development”, and so Banff National Park is not yet being managed in ways that make its ecological integrity the bottom line, rather than additional economic development.
Design and Stewardship
The design of protected areas is an important field of research in conservation biology. The essential questions involve ways of determining the best size, shape, and positioning of protected areas in order to optimize their ability to protect biodiversity, while using limited funding as efficiently as possible. The least controversial recommendations of conservation biologists are that ecological reserves should be as large and as numerous as possible. Other aspects of the design of protected areas are being actively debated, and the discussion will not be resolved until more research is undertaken. These aspects of reserve design include the following:
- The choice between size and number of protected areas: Is it preferable to have one large reserve or several smaller ones with the same total area (Figure 27.5a)? Conservation biologists identify this question with the acronym SLOSS, for single large or several small. According to ecological theory, a population in a larger reserve is expected to have a lower risk of local extinction compared with one in a smaller area. However, if separate populations occur in different reserves (even if they are relatively small), the redundancy might protect against catastrophic loss of an endangered species in a larger reserve (even if it is relatively large).
- Larger reserves have more interior habitat: So-called “interior” habitat is not influenced by environmental conditions that occur at an ecotone (or a transition between habitat types, such as a forest edge). Ecotonal habitats may be penetrated by invasive species, predators, and parasites (such as cowbirds), which can be an important problem in protected areas. In addition, many species require interior habitat for successful breeding. Larger reserves have proportionately more interior habitat, a factor that contributes to the conservation of interior species (Figure 27.5a).
- Reserves should be clumped: Similar reasoning suggests that it is better to aggregate reserves than to arrange them in a linear fashion. This would reduce the average distance between protected areas, which may enhance their ecological connections (Figure 27.5d).
- The role of corridors: A system of reserves connected by corridors of suitable habitat may provide better opportunities for gene flow and re-colonization after extirpation (Figure 27.5e). Admittedly, however, corridors might also make it easier for diseases and invasive species to spread among reserves.
- Circular reserves are better: A circle has a smaller ratio of edge to area than any other two-dimensional shape. To avoid extensive edge habitat in the design of a protected area, a roughly circular shape may be preferable (Figure 27.5b).
- Reserves should be close together: If a population is extirpated in a protected area, the chances of natural re-colonization may be improved if the species survives in a nearby reserve. Consequently, it may be better to have unconnected reserves arranged relatively close to each other, rather than far apart (Figure 27.5c).
It is important to understand that the stewardship of biodiversity requires much more than a simple declaration that a tract of natural area is henceforth to be considered “protected.” The ecological integrity of the reserves must also be monitored and management may be necessary to conserve any threatened values. For example, if a protected area supports a population of an endangered species, its abundance and habitats should be monitored. If any decline is observed, the environmental causes should be determined by research, and then, if possible, be mitigated to prevent or repair the damage. Management activities can include, among other actions, patrols to prevent the poaching of animals or timber, modification of habitats to keep them suitable for species at risk, and even captive breeding and release of endangered species.
Considered together, these stewardship actions represent an integrated program of monitoring, research, and management. The application of such a system can be illustrated by the case of the endangered Kirtland’s warbler (Dendroica kirtlandii). Monitoring has shown that this rare bird has declined in abundance, and its global population is now only a few hundred breeding pairs. Research has revealed that its only breeding habitat consists of jack pine (Pinus banksiana) stands of a particular age and structure. Many such stands have now been protected in the breeding range of the warbler. However, as these stands get older, they are no longer suitable as breeding habitat. Consequently, management is actively developing appropriate breeding habitat by prescribed burning and the planting of jack pine. Additional research has shown that the endangered warbler is heavily parasitized by the brown-headed cowbird. Consequently, cowbird populations are being controlled in the breeding habitat of the warbler. Further research and monitoring are being directed to the environmental stressors that affect the warbler during its little-known migrations and on its wintering range. Of course, these integrated activities of monitoring, research, and management must continue as long as Kirtland’s warbler remains endangered.
Canadian Focus 27.2. The Nature Conservancy of Canada Governments throughout Canada have created many protected areas, but their actions are almost exclusively on so-called Crown land, which they already own. However, many of the most important properties of conservation value are privately owned by individuals or corporations. This is particularly the case of southern regions of Canada, where most species-at-risk and endangered ecosystems occur. Because of a lack of money and other priorities, governmental agencies are often reluctant to secure ecologically important habitat on privately owned lands. For this reason, conservation charities known as land trusts have formed for the purpose of raising funds to acquire private property in order to establish protected areas.
At the national level, the largest of these organizations is the Nature Conservancy of Canada (NCC). The focus of NCC is on acquiring private property of high conservation value, which it does by purchasing or accepting donations of real estate, as well as rights of land-use. The latter involves a kind of private property called a conservation easement (or in Quebec, a servitude). If owned by NCC, a conservation easement can prevent current and future owners of the real estate from converting its natural habitat into residential lots or cultivated agriculture, or from engaging in other proscribed activities that might threaten the natural values of the property. There are many other land trusts that operate at local and provincial scales that also acquire private conservation lands and easements. However, NCC is the only national organization, and it is by far the biggest.
Since its origin in 1962, NCC has contributed to the protection of 1.1-million hectares of natural habitat throughout Canada (Freedman, 2013; NCC, 2015). In 2013-14, NCC raised (and spent) more than $87 million to advance its conservation mission. In 2012, it met the goals of its five-year “Force for Nature” fund-raising campaign, which raised more than $546 million, from the private sector and all levels of government. More than 62-thousand Canadians contribute annually to support the work of NCC.
The conservation actions of NCC are being guided by “ecoregional plans”, also known as “conservation blueprints”, which help to identify the most important places where private action can make the greatest difference in protecting native species and natural ecosystems. These plans are developed in close partnership with governments, the U.S. Nature Conservancy, Ducks Unlimited Canada, provincial and local land trusts, industrial interests, and academic scientists. As of 2015, NCC had completed 15 “ecoregional plans”, all for southern parts of Canada, which are the most imperiled from the conservation perspective. Eventually, NCC intends to have ecoregional plans for all regions of Canada. The results of the plans are used to identify focal areas for land assemblies – there are now 77 of these key areas for action, which NCC refers to as “Natural Areas,” each of which is the focus of a “Natural Area Conservation Plan.”
Successful conservation action requires more than just acquiring properties – each project must also be properly stewarded to maintain or enhance its natural values. Stewardship actions range from posting signs at property boundaries, to innovative, science-based management actions that are needed to sustain particular species or ecological communities.
For example, NCC routinely subjects its tall-grass prairie reserves in southeastern Manitoba to prescribed burns, which prevents the endangered grassland from being degraded by incursions of shrubs and trees. At NCC reserves that protect imperiled Garry oak forest on southern Vancouver Island, stewardship volunteers spend many hours pulling alien weeds that threaten rare plants. One last example involves a protected area of rare Carolinian forest at Clear Creek in southern Ontario, where NCC is converting adjacent cornfields into natural forest in order to increase the size and enhance the viability of the conserved ecosystem. These kinds of stewardship activities must be advised by leading-edge scientific knowledge, which NCC develops by hiring ecologists and by working in partnership with other organizations.
The Nature Conservancy of Canada is only one example of the many highly motivated and effective non-governmental conservation organizations in Canada. Their work is crucial to sustaining the biodiversity-at-risk of our country. Of course, being charities, private organizations like NCC can only spend money that they can manage to raise, which is a good reason for all sectors of our society to support their important work of protecting natural habitats.
International Conservation
The conservation of wild species of plants and animals is now regarded by almost all societies as a worthwhile and important objective. As a result, in most countries, many people are becoming active in support of conservation. Evidence of these hopeful changes includes the fact that governments are becoming more engaged in the conservation of indigenous and global biodiversity, while large numbers of non-governmental organizations are becoming active at local, national, and international levels. In addition, more ecologists and other scientists are conducting biodiversity-related research and training in universities and other institutions. Most importantly, many citizens are working hard to conserve biodiversity, either by taking direct action on their own property, or by supporting the worthwhile initiatives of ENGOs, private companies, and governments.
All of these activities contribute to the greater agenda of biodiversity conservation, particularly by doing the following:
- identifying and protecting the habitats of rare and endangered species and communities, while also conserving representative areas of natural ecosystems
- working to control the illegal trade in the products of endangered species, such as elephant ivory, rhino horn, bear gallbladders, tiger bones and hides, and wild ginseng (see Global Focus 27.1)
- increasing the awareness of people about biodiversity issues and the need to conserve all species, ecosystems, and ecological sevices
- conducting necessary research into the biology and ecology of endangered species and ecosystems
- raising or providing funds for all of the above
Not surprisingly, the intensity of these conservation activities is greatest, and increasing most quickly, in relatively wealthy countries, such as Canada. Those countries can more easily afford to allocate significant funding and personnel to this worthwhile cause. Increasingly, however, signs of awareness of the importance of biodiversity issues, and actions to conserve those values, are also rapidly developing in less-developed countries. This reflects changes in the attitudes of people and governments and is reinforced by lobbying and funding provided by domestic and international aid agencies and ENGOs. These changes are critically important because a large fraction of Earth’s threatened biodiversity occurs in tropical, less-developed countries.
Of course, a respect for nature has always been an integral component of major religions that developed in tropical countries, such as Buddhism, Hinduism, Jainism, and Taoism. Nevertheless, this respect has not necessarily been translated into a real-world conservation ethic among the peoples of those or any other nations. As a result, wildlife and natural habitats have suffered badly, mostly as a result of the extensive conversion of natural ecosystems into agricultural ones, but also from timber harvesting and other ways as well.
It is beyond the scope of this book to describe the many international agencies and organizations that are active in the protection of global biodiversity. Some of the most important of them are Conservation International, the International Union for the Conservation of Nature (IUCN), The Nature Conservancy (U.S.), the United Nations Environment Programme (UNEP), the World Resources Institute, the World Wildlife Fund, and the Worldwatch Institute. These organizations are active in conservation advocacy, in protecting species and natural habitat, in education and lobbying, in research and monitoring, and in raising funds for the protection of biodiversity.
To illustrate the rapid development of international conservation activities, we can examine a program known as the Global Biodiversity Strategy (Reid et al., 1992). This is a joint program of the International Union for the Conservation of Nature, the World Resources Institute, and the United Nations Environment Programme. Its broad objectives are to maintain essential ecological processes and life-support systems on Earth, to preserve biodiversity, and to ensure the sustainable development of natural resources. Although these are rather general goals, they are important because they link the conservation of biodiversity with the sustainable development of the human economy. One cannot occur without the other, a fact that must be acknowledged by any governments or agencies that support the Global Biodiversity Strategy.
Through this initiative, all nations can initiate meaningful actions to conserve and protect their biodiversity for the benefit of present and future generations of people, as well as for reasons of intrinsic value. To achieve this end, 85 specific actions are recommended for implementation by nations that have committed to the Strategy. The following five actions are considered essential:
- ratification and implementation of the recommendations of the Convention on Biological Diversity, as presented in 1992 by UNEP at the United Nations Conference on Environment and Development, held at Rio de Janeiro, Brazil (the “Earth Summit”)
- implementation of the actions detailed in the Global Biodiversity Strategy, with a focus on efforts to conserve and protect the indigenous biodiversity of signatory nations
- creation of an international administrative mechanism to ensure broad participation in decisions concerning global biodiversity, with representation from governments, the scientific community, citizens, industry, the United Nations, and non-governmental organizations
- establishment of an international network, linked to the Convention on Biological Diversity, to monitor threats to biodiversity so that individuals and organizations can be alerted and take appropriate actions
- integration of biodiversity considerations into planning processes for national development
The Convention on Biological Diversity is now the leading international treaty to advance the conservation of biodiversity, within the context of a comprehensive agenda for sustainable development (UNEP, 2015). The three key goals of the Convention are the conservation of biodiversity, the sustainable use of its components, and the equitable sharing of benefits from the use of genetic resources. The 194 ratified parties to the Convention are engaged in various co-operative initiatives and information and technology sharing, and are committed to identify and conserve their biodiversity.
A key action is to periodically undertake science assessments of the status of global biodiversity (SCBD, 2015). Broadly speaking, the most recent assessment found that the prospects of biodiversity were rapidly worsening in many parts of the world, although considerable progress was being made in co-operative planning and in the designation of protected areas and other conservation measures in many countries.
It is too soon to tell whether these international actions will be successful because the programs began only in the late 1970s (as an earlier program called the World Conservation Strategy). However, it is encouraging to know that this sort of comprehensive international effort exists and that almost all of Earth’s nations are participating, including countries in all stages of economic development. Of course, it remains to be seen how effective the individual and collective actions will be. This is substantially because, although environmental and biodiversity issues are extremely important to sustainability of the human enterprise, their resolution does not yet have much political traction in many countries, including in Canada
Conclusions
If Earth’s resources are to be used by people on an ecologically sustainable basis, rare and endangered species and threatened natural ecosystems must be protected. Clearly, an international program like the Global Biodiversity Strategy is needed to guide the process of sustainable development.
The modern predicament of extinction and endangerment of biodiversity is a critical element of the global environmental crisis, and its resolution is a key aspect of ecologically sustainable development of the human economy. Hopefully, the increasing intensity of conservation activities worldwide will be sufficient to turn the tide, so future generations will regard these ongoing actions as a “success story” of global conservation. Any alternative result would be catastrophic and tragic.
The one process . . . that will take millions of years to correct is the loss of genetic and species diversity by the destruction of natural habitats. This is the folly that our descendants are least likely to forgive us. E.O. Wilson (cited in Reid et al., 1992)
Questions for Review
- What are the greatest modern threats to global biodiversity?
- What are the greatest threats to the native biodiversity of Canada?
- List five examples of species that became extinct at any time during the past 500 years, and explain why they were lost.
- List five examples of species that were endangered but have since recovered, and explain why this happened.
Questions for Discussion
- How are the products of biodiversity important in your life? Compile a list of ways in which you routinely use products of biodiversity in your daily routine, such as foods, materials, and medicines. Are there substitutes for these uses?
- Do you know of any species that are rare or endangered in the region in which you live? Find several examples, and identify the habitat needs of those species. Do you think that these rare or endangered species are being adequately protected? What more could be done? How can you help?
- Find several examples of endangered spaces (endangered ecosystems) in your region and decide whether they are being adequately protected. What more could be done? How can you help?
- We learned in this chapter that the greatest modern threat to Earth’s biodiversity is deforestation in tropical countries. Can you think of ways in which Canadians are economically linked with deforestation in the tropics? For example, do Canadian consumers provide a demand for tropical-forest products? Do Canadians hold some of the foreign debt of tropical countries? How might these circumstances contribute to tropical deforestation?
- Everyone can help to protect endangered species and spaces. List some of the ways in which you can contribute to solving the problems of endangered biodiversity in the region where you live, in Canada, and internationally.
Exploring Issues
- Your provincial government has committed itself to ensuring that its indigenous biodiversity will be conserved. Your services have been retained to provide advice on how to accomplish that goal. What would you tell the government to do in order to identify and conserve its indigenous species and ecosystems?
References Cited and Further Reading
Beacham, W., F.V. Castronova, B. Freedman, and S. Sessine (eds.). 2001. Beacham’s Guide to International Endangered Species. Gale Group, Farmington Hills, MI.
Blockstein, D.E. and H.B. Tordoff. 1985. Gone forever: A contemporary look at the extinction of the passenger pigeon. American Birds, 39: 845-851.
Butler, R.A. 2017. Calculating Deforestation Figures for the Amazon. Mongabay.com. https://web.archive.org/web/20180614002927/https://rainforests.mongabay.com/amazon/deforestation_calculations.html
Canadian Council on Ecological Areas (CCEA). 2015. Canadian Conservation Areas Database. CCEA, Natural Resources Canada, and Environment Canada, Ottawa, ON.
Canadian Endangered Species Conservation Council (CESCC). 2014. General Status of Species in Canada. CESCC, Ottawa, ON.web.archive.org/web/20140807013023/https://www.ec.gc.ca/indicateurs-ind...n&n=37DB2E44-1
Canadian Wildlife Service (CWS). 2015. Breeding Bird Survey Results. CWS, Hull, QC. https://web.archive.org/web/20170103142354/http://www.ec.gc.ca/ron-bbs/P001/A001/?lang=e
CITES. 2015. CITES Trade Database. Cites, Geneva, Switzerland. http://trade.cites.org/
Convention on International Trade in Endangered Species of Fauna and Flora (CITES). 2015. The CITES species. http://www.cites.org/eng/disc/species.php
Committee on the Status of Endangered Wildlife in Canada (COSEWIC). 2015. Canadian Species at Risk. COSEWIC, Ottawa, ON. webarchive.bac-lac.gc.ca:8080/wayback/20140805200248/www.cosewic.gc.ca/eng/sct5/index_e.cfm
Day, D. 1989. The Encyclopedia of Vanished Species. McLaren, Hong Kong.
Diamond, J.M. 1982. Man the exterminator. Nature, 298: 787-789.
Dobson, A.P. 1996. Conservation and Biodiversity. W.H. Freeman & Co., San Francisco, CA. Eldredge, N. 1998. Life in the Balance: Humanity and the Biodiversity Crisis. Princeton University Press, Princeton, NJ.
Environment Canada. 2015a. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Environment Canada, Ottawa, ON. http://webarchive.bac-lac.gc.ca:8080/wayback/20131120172744/http://www.ec.gc.ca/cites/
Environment Canada. 2015b. Protected Areas Data. Environment Canada, Ottawa, ON. webarchive.bac-lac.gc.ca:8080/wayback/20140807013537/http://ec.gc.ca/indicateurs-indicato...n&n=8390800A-1
Erwin, D.A. 1990. The end-Permian mass extinction. Annual Reviews in Ecology and Systematics, 21: 69-91.
Feduccia, A. 1985. Catesby’s Birds of Colonial America. University of North Carolina Press, Chapel Hill, NC.
Finch, D.M. 1991. Population Ecology, Habitat Requirements, and Conservation of Neotropical Migrating Birds. USDA Forest Service General Technical Report RM-205, Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO.
Food and Agricultural Organization of the United Nations (FAO). 2015. FAOSTAT Forestry Statistics Database. FAO, Rome. http://www.fao.org/forestry/statistics/84922/en/
Freedman, B. 1995. Environmental Ecology. 2nd ed. Academic Press, San Diego, CA.
Freedman, B. 2013. A History of the Nature Conservancy of Canada, 1962-2012. Oxford University Press, Canada, Toronto, ON.
Gaston, K.J. and J.I. Spicer. 2004. Biodiversity: An Introduction. 2nd ed. Blackwell Science, Oxford, UK.
Gould, S.J. 1989. Wonderful Life: The Burgess Shale and the Nature of History. Norton, New York, NY.
Groombridge, B. and M.D. Jenkins. 2002. World Atlas of Biodiversity: Earth’s Living Resources in the 21st Century. University of California Press, Berkeley, CA.
Heywood, V.H. (executive ed.). 1995. Global Biodiversity Assessment. Cambridge University Press Cambridge, UK.
Holmes, R.T., T.W. Sherry, and F.W. Sturges. 1986. Bird community dynamics in a temperate deciduous forest: long-term trends at Hubbard Brook. Ecological Monographs, 56: 201-220.
International Union for the Conservation of Nature (IUCN). 2015. The IUCN Red List of Threatened Species. IUCN, Gland, Switzerland. http://www.iucnredlist.org/
International Whaling Commission (IWC). 2008. Whale Population Estimates. IWC, Cambridge, UK. https://iwc.int/estimate
Krausman, P.R. and J.W. Cain III (Editors). 2013. Wildlife Management and Conservation: Contemporary Principles and Practices. Johns Hopkins University Press, Baltimore, MD.
Leader-Williams, N., S.D. Albou, and P.S.M. Berry. 1990. Illegal exploitation of rhinoceros and elephant populations: Decline, law enforcement, and patrol effort in Luangwa, Zambia. Journal of Applied Ecology, 27: 1055-1087.
Martin, P.S. and H.E. Wright (eds.). 1967. Pleistocene Extinctions: The Search for a Cause. Yale University Press, New Haven, CT.
Myers, N. 1979. The Sinking Ark: A New Look at the Problem of Disappearing Species. Pergamon, Oxford.
Myers, N. 1988. Tropical forests and their species: going . . . going . . . . Pp. 28–35 in: Biodiversity (E.O. Wilson, ed.). National Academy Press, Washington, DC.
National Marine Fisheries Service (NMFS). 2007. Conservation Plan for the Eastern Pacific Stock of the Northern Fur Seal. Juneau, AK.https://web.archive.org/web/20150923022454/http://www.nmfs.noaa.gov/pr/pdfs/conservation/plan_nfs_dec2007.pdf
Nettleship, D.N. and P.G.H. Evans. 1985. Distribution and status of the Atlantic Alcidae. Pp. 54-154 in: The Atlantic Alcidae. (D.N. Nettleship and T.R. Birkhead, eds.). Academic Press, New York.
Nitecki, M.H. (ed.). 1984. Extinctions. University of Chicago Press, Chicago.
Norse, E.A. and L.B. Crowder (eds.). 2005. Marine Conservation Biology: The Science of Maintaining the Sea’s Biodiversity. Island Press, Washington, DC.
Norton, B.G. (ed.). 1986. The Preservation of Species. Princeton University Press, Princeton, NJ.
Page, J.D., Bayley, S.E., Good, D.J., Green, J.E., and Ritchie, J.P.B. 1996. Banff–Bow Valley: At the Crossroads. Summary Report. Report of the Banff-Bow Valley Task Force, Parks Canada, Ottawa, ON.
Perlman, D.L. and G. Adelson. 1997. Biodiversity: Exploring Values and Priorities in Conservation. Blackwell Science, Oxford, UK.
Peters, R.L. 1991. Consequences of global warming for biological diversity. Pp. 99-118 in: Global Climate Change and Life on Earth. (R.L. Wyman, ed.). Routledge, Chapman, & Hall, New York, NY.
Primack, R.B. 2013. Essentials of Conservation Biology. 6th ed. Sinauer, Sunderland, MA.
Protected Planet. 2015. Discover the world’s protected areas. http://www.protectedplanet.net/#0_88_-347.5_1
Raup, D.M. 1986. Biological extinctions in Earth history. Science, 231: 1528-1533.
Reaka-Kudla, M.L., D.E. Wilson, and E.O. Wilson (eds.). 1997. Biodiversity II: Understanding and Protecting Our Biological Resources. National Academy Press, Washington, DC.
Reid, W., C. Barker, and K. Miller (principal authors). 1992. Global Biodiversity Strategy. World Resources Institute, Washington, DC.
Secretariat of the Convention on Biological Diversity (SCBD). 2015. Global Biodiversity Outlook 4. SCBD, Montreal, QC. http://www.cbd.int/gbo4/
Simberloff, D. 1988. The contribution of population and community biology to conservation science. Annual Reviews in Ecology and Systematics, 19: 473-511.
Soule, M.E. (ed.) 1986. Conservation Biology: The Science of Scarcity and Diversity. Sinauer, Sunderland, MA.
Steadman, D.W. 1991. Extinction of species: Past, present, and future. Pp. 156-169 in: Global Climate Change and Life on Earth. (R.C. Wyman, ed.). Routledge, Chapman, & Hall, New York, NY.
Terborgh, J. 1989. Where Have All the Birds Gone? Princeton University Press, Princeton, NJ.
United Nations Environment Program (UNEP). 2015. Convention on Biodiversity. Montreal, QC. http://www.cbd.int/
United Nations Environment Program (UNEP). 2015. Environmental Data Explorer. http://ede.grid.unep.ch/
Vitousek, P.M. 1988. Diversity and biological invasions of oceanic islands. Pp. 181-189 in. Biodiversity (E.O. Wilson, ed.). National Academy Press, Washington, DC.
Wilson, E.O. (ed.). 1988. Biodiversity. National Academy Press, Washington, DC.
World Bank. 2015. Terrestrial protected areas (% of total land area). World Bank, Geneva, Switzerland. http://data.worldbank.org/indicator/ER.LND.PTLD.ZS


