4.1: Fossil Fuels and Products of Combustion
- Page ID
- 47171
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fossil Fuel Elements
Fossil fuels are hydrocarbons comprised primarily of the following elements: carbon and hydrogen and some sulfur, nitrogen, oxygen, and mineral matter. Mineral matter turns into ash when burnt.
The composition and the amounts of these elements change for different fossil fuels (coal, petroleum, and natural gas), but the elements are the same, as shown in Figure 4.1.1. For example, there is more hydrogen in liquid fuels than in coal per unit mass.
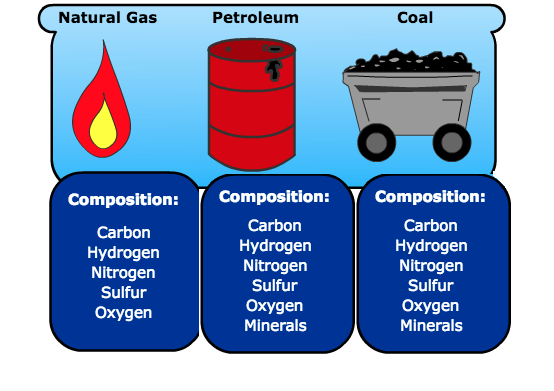
Figure 4.1.1. Fossil fuel composition
Combustion is rapid oxidation of the fossil fuel’s elements resulting in the generation of heat. When these elements oxidize (or combine with oxygen), products of combustion are formed.
Products of Combustion
Some of the fuel (hydrocarbon) may not completely burn during combustion and therefore is released into the atmosphere along with the products. The products that are formed during combustion of fossil fuels are shown in Figure 4.1.2.
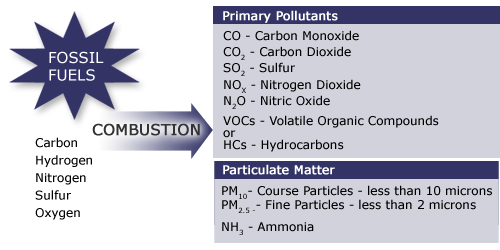
Figure 4.1.2. Products formed during combustion of fossil fuels
We will now look at six products of combustion:
- Carbon Dioxide
- Carbon Monoxide
- Sulfur Dioxide
- Nitrogen Oxides
- Lead
- Particulate Matter
Carbon dioxide (CO2)
Carbon dioxide is the principal product of combustion of fossil fuels since carbon accounts for 60–90 percent of the mass of fuels that we burn.
China has emerged as the largest single emitter of energy-related CO2 emissions, reaching an all-time high of 8320 million metric tons of carbon dioxide in 2010. The United States emitted about 5610 million metric tons in 2010. Figure 4.1.3 shows the trend in carbon dioxide emissions from 1980. For Asia and Oceania, and particularly for China and India, emissions can be seen to have increased significantly in the past two decades.
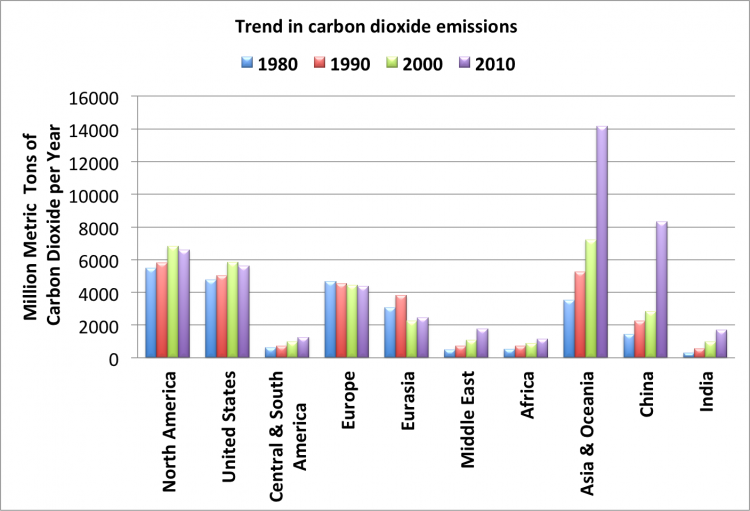
Figure 4.1.3. Graph of CO2 emission trends
Each of the end sectors (users) and their contribution (%) to the overall CO2 emissions is shown in Table 4.1.1.
Table 4.1.1. Energy-related CO2 emissions (million metric tons) by source and sector for the U.S. in 2011
| Sources | Residential | Commercial | Industrial | Transportation | Electric Power | Source Total |
|---|---|---|---|---|---|---|
| Coal | 1 | 5 | 151 | 0 | 1,718 | 1,874 |
| Natural gas | 256 | 171 | 419 | 39 | 411 | 1,296 |
| Petroleum | 78 | 49 | 345 | 1,802 | 25 | 2,299 |
| Other | 11 | 11 | ||||
| Electricity | 827 | 767 | 567 | 4 | ||
| Sector total | 1,162 | 992 | 1,482 | 1,845 | 2,166 | 5,481 |
Credit: US Energy Information Administration
Additional Information
In 2010, 41.9% of U.S. fossil-fuel emissions came from the consumption of petroleum products, and coal usage accounted for 35.4% of U.S. fossil-fuel CO2 emissions. About 22.5% of the CO2 emissions were a result of natural gas use.
Carbon Monoxide (CO)
Carbon monoxide, or CO, is a colorless, odorless gas that is formed when carbon in fuel is not burned completely. Figure 4.1.4 shows the contribution of various sources to the emissions of CO.
Figure 4.1.4. Sources of CO emissions
Carbon monoxide is a component of motor vehicle exhaust, which contributes about 55 percent of all CO emissions nationwide. Other non-road engines and vehicles (such as construction equipment and boats) contribute about 22 percent of all CO emissions nationwide. Higher levels of CO generally occur in areas with heavy traffic congestion. In cities, 85 to 95 percent of all CO emissions may come from motor vehicle exhaust.
Other sources of CO emissions include industrial processes (such as metals processing and chemical manufacturing), residential wood burning, as well as natural sources such as forest fires. Woodstoves, gas stoves, cigarette smoke, and unvented gas and kerosene space heaters are sources of CO indoors.
The highest levels of CO in the outside air typically occur during the colder months of the year when inversion conditions are more frequent. An inversion is an atmospheric condition that occurs when the air pollutants are trapped near the ground beneath a layer of warm air.
Sulfur Dioxide (SO2)
Sulfur dioxide, or SO2, belongs to the family of sulfur oxide gases (SOx). These gases dissolve easily in water. Sulfur is prevalent in all raw materials, including crude oil, coal, and ores that contain common metals, such as aluminum, copper, zinc, lead, and iron.
SOx gases are formed when fuel containing sulfur, such as coal and oil, is burned, and when gasoline is extracted from oil, or metals are extracted from ore. A distribution of the emissions is shown in Figure 4.1.5. SO2 dissolves in water vapor to form acid and interacts with other gases and particles in the air to form sulfates and other products that can be harmful to people and their environment.
Figure 4.1.5. Sources of SO2 emissions
Nitrogen Oxides (NOx)
Nitrogen oxides, or NOx, is the generic term for a group of highly reactive gases, all of which contain nitrogen and oxygen in varying amounts. Many of the nitrogen oxides are colorless and odorless.
Nitrogen oxides form when fuel is burned at high temperatures, as in a combustion process. The primary sources of NOx are motor vehicles, electric utilities, and other industrial, commercial, and residential sources that burn fuels as shown in Figure 4.1.6.
Figure 4.1.6. Sources of NOx emissions
Although many of the nitrogen oxides are colorless and odorless, one common pollutant, nitrogen dioxide (NO2) along with particles in the air can often be seen as a reddish-brown layer over many urban areas, as shown in Figure 4.1.7.

Figure 4.1.7. Smog over Los Angeles
Lead (Pb)
The major sources of lead emissions have historically been motor vehicles (such as cars and trucks) and industrial sources.
Due to the phase-out of leaded gasoline, metals processing is the major source of lead emissions to the air today. The highest levels of lead in air are generally found near lead smelters (devices that process lead ores). Other stationary sources are waste incinerators, utilities, and lead-acid battery manufacturers. Figure 4.1.8 shows numerous boxes of lead.

Figure 4.1.8. Lead is a metal found naturally in the environment as well as in manufactured products
Fun Fact
Lead is used in the manufacturing of many items, including glass, rubber, paint, batteries, insecticides, plumbing and protective shielding for X-rays.
Particulate Matter (PM)
Particulate matter (PM) is the general term used to describe a mixture of solid particles and liquid droplets found in the air. Some particles are large enough to be seen as dust or dirt. Others are so small they can be detected only with an electron microscope.
Different sizes of particles include:
- PM 2.5 describes the “fine” particles that are less than or equal to 2.5 µm (micro meter) in diameter.
- “Coarse fraction” particles are greater than 2.5 µm, but less than or equal to 10 µm in diameter.
- PM 10 refers to all particles less than or equal to 10 µm in diameter (about one-seventh the diameter of a human hair). PM can be emitted directly or formed in the atmosphere.
Different sources of particles include:
- "Primary" particles are formed from combustion sources and are emitted directly into the atmosphere. Examples of primary particles are dust from roads or black carbon (soot).
- "Secondary" particles are formed in the atmosphere from primary gaseous emissions. Examples of secondary particles are sulfates formed from SO2 emissions from power plants and industrial facilities; nitrates formed from NOx emissions from power plants, automobiles, and other combustion sources; and carbon formed from organic gas emissions from automobiles and industrial facilities.
Additional Information
The chemical composition of PM depends on location, time of year, and weather. Generally, primary particles make up coarse PM and secondary particles make up most of fine PM.
Health and Environmental Effects of Primary Pollutants
The pollutants that are emitted directly from a combustion process – or the products of combustion - are called “primary pollutants.” We just described these products earlier in the lesson, now we will look at their impact on the environment and human health.
Carbon dioxide (CO2)
Carbon dioxide (CO2) is not a pollutant that would harm our health but it is a proven greenhouse gas. It has an ability to absorb infrared radiation that is escaping from the surface of the earth causing the atmosphere to warm up. Excessive emission of CO2 along with other greenhouse gases is thought to contribute to the undesirable climate change.
Carbon Monoxide (CO)
As we learned earlier, Carbon monoxide, or CO, is a colorless, odorless and tasteless gas that is formed when carbon in fuel is not burned completely.
At much higher levels of exposure not commonly found in ambient air, CO can be poisonous, and even healthy individuals can be affected. Exposure to elevated levels of CO may result in:
- Visual impairment
- Reduced work capacity
- Reduced manual dexterity
- Poor learning ability
- Difficulty in performing complex tasks
The health threat from levels of CO sometimes found in the ambient air is most serious for those who suffer from cardiovascular disease such as angina pectoris.
Additional Information
In the human body, hemoglobin (an iron compound) in the blood carries the oxygen (O2) from the lungs to various tissues and transports back carbon dioxide (CO2) to the lungs. Hemoglobin has 240 times more affinity toward CO than it does for O2. Therefore, when the hemoglobin reacts with CO, it reduces the hemoglobin that is available for the transport of O2. This in turn reduces oxygen supply to the body's organs and tissues.
Sulfur Dioxide (SO2)
High concentrations of SO2 can result in the following health problems:
- Short term exposure
- Adults and children with asthma who are active outdoors will experience temporary breathing impairment.
- Individuals with asthma may experience breathing difficulties with moderate activity and may exhibit symptoms such as wheezing, chest tightness, or shortness of breath.
- Long term exposure
- Aggravation of existing cardiovascular disease
- Respiratory illness
- Alterations in the lungs’ defenses
- The subgroups of the population that may be affected under these conditions include individuals with heart or lung disease, as well as the elderly and children.
The following video describes how the body reacts to different types of air.
Together, SO2 and NOx (discussed on the following section) are the major precursors to acidic deposition (acid rain), which is associated with the acidification of soils, lakes, and streams and accelerated corrosion of buildings and monuments. We will talk more about this in the next section. SO2 also is a major precursor to PM 2.5, which is a significant health concern, and a main contributor to poor visibility.
Nitrogen Oxides (NOx)
Nitric oxide (NO) and nitrogen dioxide (NO2) together are represented by NOx. Most of the emissions from combustion devices (approximately 90%) are in the form of NO.
NOx react in the air to form ground-level ozone and fine particulates, which are associated with adverse health effects.
- Short-term exposures (e.g., less than 3 hours) to low levels of NO2 may lead to changes in airway responsiveness and lung function in individuals with preexisting respiratory illnesses. These exposures may also increase respiratory illnesses in children.
- Long-term exposures to NO2 may lead to increased susceptibility to respiratory infection and may cause irreversible alterations in lung structure.
NOx contributes to a wide range of environmental effects directly and when combined with other precursors in acid rain and ozone.
- Increased nitrogen inputs to terrestrial and wetland systems can lead to changes in plant species composition and diversity.
- Direct nitrogen inputs to aquatic ecosystems such as those found in estuarine and coastal waters (e.g., Chesapeake Bay) can lead to eutrophication (a condition that promotes excessive algae growth, which can lead to a severe depletion of dissolved oxygen and increased levels of toxins harmful to aquatic life).
- Nitrogen, alone or in acid rain, also can acidify soils and surface waters.
Additional Information
Acidification of soils causes the loss of essential plant nutrients and increased levels of soluble aluminum that are toxic to plants. Acidification of surface waters creates conditions of low pH and levels of aluminum that are toxic to fish and other aquatic organisms. NOx also contributes to visibility impairment.
Particulate Matter (PM)
Particles smaller than or equal to 10 µm (micro meter or millionth of a meter) in diameter can get into the lungs and can cause numerous health problems. Inhalation of these tiny particles has been linked with illness and death from heart and lung disease. Various health problems have been associated with long-term (e.g., multi-year) exposures to these particles. Shorter-term daily and potentially even shorter term peak (e.g., 1-hour) exposures to these particles can also be associated with health problems.
Particles can aggravate respiratory conditions, such as asthma and bronchitis, and have been associated with cardiac arrhythmias (heartbeat irregularities) and heart attacks. People with heart or lung disease, the elderly, and children are at highest risk from exposure to particles.
Particles of concern can include both fine and coarse-fraction particles, although fine particles have been more clearly linked to the most serious health effects.
- Particles larger than 2 micro meters (µm) do not penetrate beyond the nasal cavity or trachea.
- Particles smaller than 0.1 µm tend to deposit in tracheobronchia tree and are removed when exhaling.
- Particles between 0.1 and 2.0 µm penetrate deep into the lungs and settle in respiratory bronchioles or alveolar sacs.
Additional Information
In addition to health problems, PM is the major cause of reduced visibility in many parts of the United States by scattering and absorbing some of the light emitted or reflected by the body reducing the contrast. Airborne particles can also impact vegetation and ecosystems, and can cause damage to paints and building materials.
The following video describes how the body reacts to different sizes of PM.
Lead (Pb)
Exposure to lead occurs mainly through inhalation of air and ingestion of lead in food, water, soil, or dust. It accumulates in the blood, bones, and soft tissues and can adversely affect the kidneys, liver, nervous system, and other organs.
- Excessive exposure to lead may cause neurological impairments such as seizures, mental retardation, and behavioral disorders.
- Even at low doses, lead exposure is associated with damage to the nervous systems of fetuses and young children, resulting in learning deficits and lowered IQ.
- Recent studies indicated that lead may be a factor in high blood pressure and subsequent heart disease.
- Lead can also be deposited on the leaves of plants, presenting a hazard to grazing animals and to humans through ingestion.
The following video details the impact of using unleaded rather than leaded gasoline.
Secondary Pollutants
The pollutants that are emitted directly from a combustion process are called “primary pollutants.” When emitted into the atmosphere, these primary pollutants combine with other reactants and form “secondary” pollutants.
An example of a secondary pollutant would be ozone. When hydrocarbons are emitted and they react with NOx in presence of sunlight, they form ozone. Health and environmental effects of secondary pollutants are discussed in the next section.
Fun Fact
Ozone is a form of oxygen and also a poisonous gas. However, when in the earth's atmosphere, ozone acts as a protective shield against ultraviolet radiation in space.


