2.1: Energy from Burning Hydrocarbons
- Page ID
- 24086
Our understanding of the world is built on layers of abstractions. Consider the idea of a fluid. At the bottom of the abstraction hierarchy are the actors of particle physics: quarks and electrons. Quarks combine to build protons and neutrons. Protons, neutrons, and electrons combine to build atoms. Atoms combine to build molecules. And large collections of molecules act, under many conditions, like a fluid.
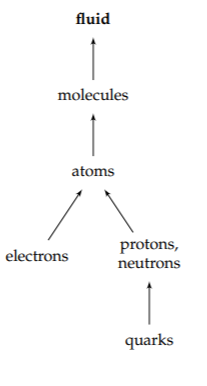
The idea of a fluid is a new unit of thought. It helps us understand diverse phenomena, without our having to calculate or even know how quarks and electrons interact to produce fluid behavior. As one consequence, we can describe the behavior of air and water using the same equations (the Navier–Stokes equations of fluid mechanics); we need only to use different values for the density and viscosity. Then atmospheric cyclones and water vortices, although they result from widely differing sets of quarks and electrons and their interactions, can be understood as the same phenomenon.
A similarly powerful abstraction is a chemical bond. We’ll use this abstraction to estimate a quantity essential to our bodies and to modern society: the energy released by burning chains made of hydrogen and carbon atoms (hydrocarbons). A hydrocarbon can be abstracted as a chain of CH2 units:
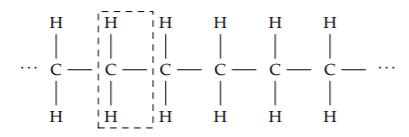
Burning a CH2 unit requires oxygen (O2) and releases carbon dioxide (CO2), water, and energy:
\[CH_{2} + \frac{3}{2}O_{2} \rightarrow CO_{2} + H_{2}O + energy\]
For a hydrocarbon with eight carbons—such as octane, a prime component of motor fuel—simply multiply this reaction by 8:
\[(CH_{2})_{8} + 12 O_{2} \rightarrow 8 CO_{2} + 8 H_{2}O + lots \: of \: energy.\]
(The two additional hydrogens at the left and right ends of octane are not worth worrying about.)
How much energy is released by burning one CH2 unit?
To make this estimate, use the table of bond energies. It gives the energy required to break (not make) a chemical bond--for example, between carbon and hydrogen. However, there is no unique carbon-hydrogen (C-H) bond. The carbon-hydrogen bonds in ethane. To make a reusable idea, we neglect those difference--placing them below our abstraction barrier--and make an abstraction called the carbon-hydrogen bond. So the table, already in its first column, is built on an abstraction.
bond energy
| \(\frac{kcal}{mol}\) | \(\frac{kJ}{mol}\) | \(\frac{eV}{bond}\) | |
| C-H | 99 | 414 | 4.3 |
| O-H | 111 | 464 | 4.8 |
| C-C | 83 | 347 | 3.6 |
| C-O | 86 | 360 | 3.7 |
| H-H | 104 | 435 | 4.5 |
| C-N | 73 | 305 | 3.32 |
| N-H | 93 | 389 | 4.0 |
| O=O | 119 | 498 | 5.2 |
| C=O | 192 | 803 | 8.3 |
| C=C | 146 | 611 | 6.3 |
| N≡N | 226 | 946 | 9.8 |
The second gives the bond energy in kilocalories per mole of bonds. A kilocalorie is roughly 4000 joules, and a mole is Avogadro’s number(6×1023) of bonds. The third column gives the energy in the SI units used by most of the world, kilojoules per mole. The final column gives the energy in electron volts (eV) per bond. An electron volt is 1.6× 10−19 joules. An electron volt is suited for measuring atomic energies, because most bond energies have an easy-to-grasp value of a few electron volts. I wish most of the world used this unit!
Let’s tabulate the energies in the combustion of one hydrocarbon unit.
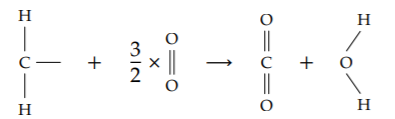
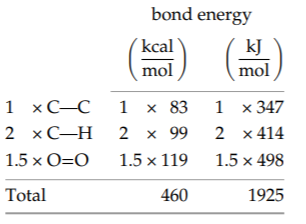
The left side of the reaction has two carbon-hydrogen bonds, 1.5 oxygen-oxygen double bonds, and one carbon-carbon bond (connecting the carbon atom in the CH2 unit to the carbon atom in a neighboring unit). The total, 460 kilocalories or 1925 kilojoules per mole, is the energy required to break the bonds. It is an energy input, so it reduces the net combustion energy.
The right side has two carbon-oxygen double bonds and two oxygen-hydrogen bonds. The total for the right side, 606 kilocalories or 2535 kilojoules per mole, is the energy released in forming these bonds. It is the energy produced, so it increases the net combustion energy.
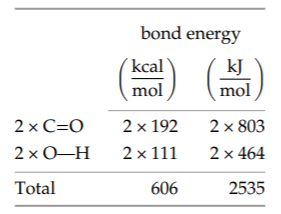
The net result is, per mole of CH2, an energy release of 606 minus 460 kilocalories, or approximately 145 kilocalories (610 kilojoules). Equivalently, it is also about 6 electron volts per CH2 unit—about 1.5 chemical bonds’ worth of energy. The combustion energy is also useful as an energy per mass rather than per mole. A mole of CH2 units weighs 14 grams. Therefore, 145 kilocalories per mole is roughly 10 kilocalories or 40 kilojoules per gram. This energy density is worth memorizing because it gives the energy released by burning oil and gasoline or by metabolizing fat (even though fat is not a pure hydrocarbon)
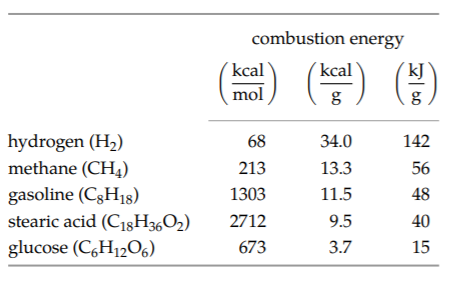
The preceding table, adapted from Oxford University’s “Virtual Chemistry” site, gives actual combustion energies for plant and animal fuel sources (with pure hydrogen included for fun). The penultimate entry, stearic acid, is a large component of animal fat; animals store energy in a substance with an energy density comparable to the energy density in gasoline—roughly 10 kilocalories or 40 kilojoules per gram. Plants, on the other hand, store energy in starch, which is a chain of glucose units; glucose has an energy density of only roughly 4 kilocalories per gram. This value, the energy density of food carbohydrates (sugars and starches), is also worth memorizing. It is significantly lower than the energy density of fats: Eating fat fills us up much faster than eating starch does.
How can we explain the different plant and animal energy-storage densities?
Plants do not need to move, so the extra weight required by using lower-density energy storage is not so important. The benefit of the simpler glucose metabolic pathway outweighs the drawback of the extra weight. For animals, however, the large benefit of lower weight outweighs the metabolic complexity of burning fats.
Exercise \(\PageIndex{1}\): Estimating the energy density of common foods
In American schools, the traditional lunch is the peanut-butter-and-jelly sandwich. Estimate the energy density in peanut butter and in jelly (or jam).
Exercise \(\PageIndex{2}\): Peanut butter as fuel
If you could convert all the combustion energy in one tablespoon (15 grams) of peanut butter into mechanical work, how many flights of stairs could you climb?
Exercise \(\PageIndex{3}\): Growth of grass
How fast does grass grow? Is the rate limited by rainfall or by sunlight?


