Iron
- Page ID
- 356
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Of all the materials used in the world today, iron is among the most common. Being the 26th element on the periodic table, it lies within the transition metals giving it special properties for structural and mechanical applications. Tracing its origins to the Middle East - most commonly believed to be in Anatolia in modern day Turkey[6] - widespread use of iron began during the middle Bronze Age. Its use in tools and weaponry did not surface until 1200 BCE when smelting, the process of extracting a metal from its ore by heating and melting it, made it a manageable material for metal workers to use. Thus, the Iron Age began. Thousands of years later, the importance of iron skyrocketed during the Industrial Revolution. Its low cost, sturdiness, and the ease of mass production mad it an ideal choice for transportation, mining, and construction. Following the industrial Revolution and during the Second World War, Iron was mass produced by the thousands of tons for ships [7].

"Iron electrolytic and 1cm3 cube" by Alchemist-hp (talk), distributed under a CC-BY 2.0 license
Chemical Properties of Iron
Iron (atomic symbol: Fe; condensed electron configuration: [Ar]4s23d6 and an electron box configuration as seen in the figure above) exhibits typical metallic behavior. Bulk iron has a lustrous silver-gray appearance and has high thermal and electrical conductivity. Iron has four naturally occurring isotopes with 56Fe being the most abundant and most common in the universe. It is the fourth most abundant element on Earth with a 4.7 mass percent distribution compared to 18 of the most common elements. The most common oxidation state for iron are +2 and +3 which are reactive with oxidizing agents. Iron has a smaller atomic radius of 126pm as compared to other transition metals. Its ionization energies (IE) range from 7.87eV/atom in the first IE, 16.87eV/atom in the second, to 30.65eV/atom in the third. It has a density of 7.86g/cm3, melting temperature of 1530ºC and a specific heat capacity of 0.45J/gºC. Ranging from 4.67-4.81eV, iron has a moderate to high work function, which is the energy required to remove an electron from the valence band.[8]
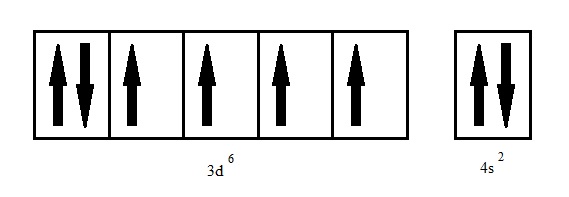
Electron box configuration of an isolated iron atom. Iron has four unpaired electrons in the 3d-orbital.
Magnetic Properties of Iron
Ferromagnetism
Characteristic of transition metals, iron has unpaired electrons in its 3d-orbital. Thus, it exhibits a magnetic property called ferromagnetism. Above a certain temperature, known as the Curie temperature (TC = 770°C [4]), ferromagnetic iron becomes paramagnetic. In the absence of a magnetic field, the domains in iron are randomly oriented and thus the material has a net magnetization of zero. Iron has a large permanent magnetization and large positive magnetic susceptibility even in the absence of an applied field. Once a field is applied to the iron, the domains align parallel to the applied magnetic field. The domains that are parallel or nearly parallel are allowed to grow in the direction of the applied field. [2],[4]
For crystalline iron, the electrons in the 3d-orbital parallel their spins and occupy different wave functions[4]. The potential energy of the overall crystal is reduced due to the exchange energy interaction which is a compromise between the Pauli Exclusion Principle and the electrostatic interaction energy. This lower potential energy is more energetically favorable and thus the majority of the 3d electrons will spontaneously align their spins parallel to each other.[4]
Ferrimagnetism
The oldest known magnetic ore is ferrimagnetic iron ferrite. Iron ferrites, such as Fe3O4, exhibit similar behavior as ferromagnetic materials. As in ferromagnetic materials, there is an overall magnetization in the direction of the applied field although some of the spin of shorter magnitude are opposing the field decreasing the overall magnetization.[4]
Saturation Magnetization
Saturation magnetization is when all of the atomic magnetic moments have been aligned as much as possible. Iron crystal has a magnetic spin of 2.2 Bohr Magnetons aligning in the same direction to give a magnetic field of 2.2T [4]. Saturation magnetization is heavily temperature dependent. If the temperature is increased, thermal agitation disrupts the alignment of the spins. Each magnetic domain is individually magnetized to saturation. [2],[4]
Hysteresis loops plot the induced field vs. the applied field. The point at which the loop stops increasing is the positive saturation magnetization. Soft magnetic materials have narrow hysteresis loops while hard magnetic materials have wider hysteresis loops. [2]
Domains
Heating iron to its Curie temperature and then cooling it will allow for the iron crystal to have no net magnetization. This is due to the formation of domains in random orientations within the crystal that form in such a way as to cancel the magnetization. The material inherently has magnetostatic energy. Division of a single domain that creates a magnetic field outside of the domain reduces the overall external field by having opposing field lines in neighboring domains. This works to reduce the magnetostatic energy.[4]
Division into many domains is energetically favorable because the magnetostriction energy of the field created as a result of the electron spins has to be minimized in order to ahcieve an optimal domain size (approximately 1-100μm for iron [2]). This optimal domain size is reached when a compromise between magnetostatic energy supporting antiparallel alignment and the exchange energy supporting parallel spin is reached. It is more energetically favorable to rotate the spins over many atomic distance rather than one. The region in which the rotation occurs is known as the Bloch wall. The movement within the walls does not carry on continuously, rather, it occurs in discrete motions, a process which is known as the Barkhausen effect. The movement of spins across domains can be impeded by particles of a second phase, cracks, dislocations and stresses caused by grinding, &etc. [2]
Mechanical Properties of Iron
The mechanical properties of iron make it ideal for structural and mechanical applications. Yield strength (σY) is the point at which a material begins to plastically deform. High-carbon steels, low alloy steels, low-carbon steels, and cast iron have the highest yield strength among structural materials with high-carbon steels having the highest yield strength of 400-1155MPa. Ultimate tensile strength (σUTS) is the maximum stress a material sample can withstand before the cross-sectional area begins to decrease. Stainless steels have the highest range of tensile strength 480-2240MPa. Young's modulus of elasticity (E) is an indicator of strength of a material. Steels have the highest Young's modulus of all common structural materials ranging from 189-217GPa. Ferrous alloys rank among the highest in thermal conductivity (λ) with low-carbon steels ranging from 49-54W/(mK). As temperature increases, thermal vibrations of the atoms increases as does the average separation distance of adjacent atoms. This is measured by the linear thermal expansion coefficient (α). Ideally, the thermal expansion coefficient should be similar to other materials that are being fused together. Ferrous alloys have similar linear thermal expansion coefficient values. These values of iron, iron alloys and other common structural materials are in the Comparative Table. [1],[3]

Comparative Table of common structural materials. The yield stress is replaced by compressive stress in ceramics.
Common Ferrous Alloys
The most common engineering materials are ferrous alloys (constituting up to 90% by weight). There are two categories of ferrous alloys based on the amount of carbon present in the alloy composition. The first carbon-containing alloy is steel, typically containing 0.05-2.0 wt % of carbon. Within this first category, it is important to distinguish between low-alloy and high-alloy steels. Low-alloy steels contain less than 5 wt % total of non-carbon alloying material. These alloys are relatively inexpensive and are highly ductile and thus easily formed. These properties alone make low-alloy steels an ideal choice for a variety of uses. [5]
High-alloy steels contain greater than 5 wt % total non-carbon additions. Although more expensive than low-alloy steels, the use of high-alloy steels is justifiable for achieving certain applications that require different properties suited to that application. High-alloy steels are used in stainless steels resistant to rusting and staining (because of the chromium in the alloy) which is a highly desired property for structural applications. Other applications of stainless steels are spring and cutlery. Tool steels are high alloys whose composition is ideal for machining applications. The composition of tool steel is dependent on the function of the tool being made: wheter it will be used for cutting, forming or otherwise shaping another material. The final (and most expensive) high-alloy steels are superalloys which provide stability and heat-resistance in high-temperature applications. [5]
The second carbon-containing alloys are cast irons with 2.0-4.5 wt. % of carbon. These alloys have relatively low melting temperatures and low liquid-phase viscosities. Cast iron is formed into its desired shape by melting the alloy and pouring it into a mold. It retains the shape of the mold by solidifying. There are four types of cast irons. White iron has its ductile properties increased by a factor of 20 and a strength that is double than that of steel. Lastly, malleable iron with reasonable ductility is formed first by casting white iron and then heat-treating to produce graphite precipitates. [5]
A final category of ferrous alloys is rapidly solidified alloys. The amorphous metal structure in this alloy optimizes strength, toughness, and corrosion resistance. This technique of solidifying alloys is relatively new and the focus of research and development. [5]
Composition of Core Materials
Iron and its alloys are commonly used as core materials in solenoids and other magnetic and electronic devices and applications. Low carbon steel has a small permeability with the disadvantage of having higher core power losses than grain-oriented iron alloys. Purification of iron is costly but does improve the permeability of iron with the disadvantage of increasing eddy-current losses. [2]
Iron-silicon alloys have higher permeability than low carbon steels and experience less eddy-current losses. Heat treatment of these alloys can occur at higher temperatures with less losses due to phase changes during cooling. Increasing the amount of silicon alloy in the material decreases the amount of core material used but above a certain silicon composition ( about 4-5wt.%), the alloy becomes too brittle to roll. [2]
Alnico is a hard magnetic material composed of varying amounts of aluminum, nickel, iron, cobalt and other minor constituent materials. The properties of Alnico are optimized by using certain heat treatments to achieve the preferred grain orientation. The optimal grain orientation is that which is parallel to the [100] direction (i.e., the easy direction). Rod-shaped iron and cobalt-rich precipitates are formed along these direction which are considered to be small, single domain particles imbedded within the Alnico aluminum and nickel based matrix.[2]
Pressing and sintering ceramic ferrite materials along the easy axis allows for the crystallization of these materials to arrange parallel to each other leading to brittle and relatively inexpensive materials. [2]
Applications
Soft Magnetic Materials
If the area within the hysteresis loop is narrow, the material is a soft magnet. There must be a compromise between having the highest possible permeability while also being easily magnetized and demagnetize [2]. Typically, soft magnets are used where repeated cycles of magnetization and demagnetization are wanted and also in application which require low hysteresis losses. Ideally, soft magnets would have zero or near zero coercive fields, which is the magnetizing field required to completely demagnetize a sample material.[4]
Silicon-steels have increase resistivity and reduced eddy current losses. These are typically used in power transformer and electric machinery. Nickel-iron alloys have low coercive fields, low hysteresis losses, and high permeability making them useful alloys in low magnetic field applications. Iron ferrites are ideal magnetic materials used in high frequency electronic applications. [4]
Eddy currents introduce power loss into soft magnetic material applications. This particular power loss is combated in part by introducing lamella into the design. There is, however, still some eddy current losses existent within the laminations themselves but this only constitutes about 1% of the power loss . [2]
Grain orientations along the easy direction are preferred. The easy direction is determined by magnetic anisotropy and in the case of iron is along the [100] direction [4] and is usually the direction in which spin magnetic moments will be spontaneously aligned in a demagnetized state [2].
Hard Magnetic Materials
Hard magnetic materials are hard to magnetize and demagnetize a property useful in memory recording and memory storage. Ideally, hard magnetic materials have very large coercive fields and can store large amounts of energy. Barium ferrite (BaFe12O9) and strontium ferrite (SrFe12O9) are ceramic magnets considered hard magnets. Newer hard magnetic materials are neodymium-iron-boron (NdFeB) with a Curie temperature of 300°C and Alnico with a Curie temperature of about 700°C. [4]
Additional Applications
- Ferrite powder imbedded in plastic materials yield flexible magnets used in applications such as gaskets in refrigerator doors. [2]
- Fe2O3 has applications in recording tapes, disks, drums and magnetic strips on credit cards.[2]
- Bubble domain memory was researched heavily while its use was widespread. A single domain in the material constitutes one bit of stored information. [2]
Production Methods
The production of iron and its alloys involves three main components: limestone, coke (bituminous coal) and iron-ore. These materials are placed into the top of a blast furnace which is a large steel cylinder lined with brick. The melted concoction is blasted into the furnace through nozzles. The main reaction is between carbon and oxygen to form carbon monoxide which then reacts with the iron oxide and reduces it to iron. The molten metal sinks to the bottom of the furnace while the impurities float to the top. After a certain period, the molten metal is funneled out from below into carts. The molten metal is then used to make iron and steel ingots. The ingots can then be refined into different forms of steel, rolled into shapes, cast and/or forged. [3]
Heat treating a material modifies its microstructure which can optimize desired properties of that material for a different applications. Iron and steel can exist in many phases and lattice structures at different temperatures. At a temperature of 727ºC, it exist as a body-centered cubic solid solution known as α-ferrite, or simply ferrite. Its intersticialcies are carbon atoms, although only a very small amount of carbon can dissolve into the BCC structure. In this phase, it is relatively soft and ductile. Ferrite is magnetic from room temperature to about 768ºC. Following this is austenite with a face-centered cubic structure. FCC has a higher solid solubility and can, therefore, contain more carbon atoms. Austenite is ductile at high temperatures and has good formability. Lastly, there is cementite, otherwise known as carbide. Carbide is hard and brittle and contains properties significant to steel.[5]
References
- Ashby, M. Materials Selection in Mechanical Design, 4th Edition. London, United Kingdom: Butterworth-Heinemann 2013.
- Hummel, R. Electronic Properties of Materials, 2nd Edition, New York: Springer-Verlag 1992.
- Kalpakjian, S.; Schmid, S, Manufacturing Engineering and Technology, 4th Edition. Upper Saddle River, NJ:Prentice Hall, 2001
- Kasap, S.O. Principles of Electronic Materials and Devices, 2nd Edition. New York, NY: McGraw-Hill, 2002.
- Shackelford, J. Introduction to Materials Science for Engineers, 6th Edition. Upper Saddle River, NJ: Prentice Hall, 2004.
- Spoerl, J. A Brieft History of Iron and Steel Production. Saint Anselm College. Web.6 Dec. 2015.
- N/A. World War II In the San Francisco Bay Area: A National Register of Historic Places Travel Itinerary. National Park Service. Web. 8 Dec. 2015.
- Zumdahl, S; Zumdahl, S. Chemistry, 8th Edition. Belmont, CA: Cengage, 2010.

