35.4: Uses of Jominy Data
- Page ID
- 34951
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Measurement of Hardenability
Data from the Jominy end quench test can be used to determine whether a particular steel can be sufficiently hardened in different quenching media, for different section diameters.
For example, the cooling rate at a distance of 9.8 mm from the quenched end is equivalent to the cooling rate at the centre of an oil-quenched bar with a diameter of 28 mm. Full transformation to martensite in the Jominy specimen at this position indicates that a 28 mm diameter bar can be through hardened, i.e. hardened through its full thickness.
A high hardenability is required for through hardening of large components. This data can be presented using CCT (Continuous Cooling Transformation) diagrams which are used to select steels to suit the component size and quenching media. Slow quenching speeds are often chosen to reduce distortion and residual stress in components.
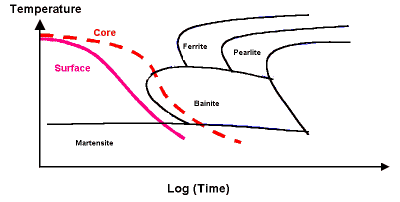
CCT diagram for an alloy steel
Slower cooling rates occur at the core of larger components, compared to the faster cooling rate at the surface. In the example here, the surface will be transformed to martensite, but the core will have a bainitic structure with some martensite.
Effects of Alloying and Microstructure
Jominy end quench test can also be used to demonstrate the effects of microstructure and alloying variables on the hardenability of steels. These include alloying elements and grain size.
Alloying elements.
The main alloying elements which affect hardenability are carbon, boron and a group of elements including Cr, Mn, Mo, Si and Ni.
Carbon
Carbon controls the hardness of the martensite. Increasing the carbon content increases the hardness of steels up to about 0.6wt%. At higher carbon levels, the formation of martensite is depressed to lower temperatures and the transformation from austenite to martensite may be incomplete, leading to retained austenite. This composite microstructure of martensite and austenite gives a lower hardness to the steel, although the microhardness of the martensite phase itself is still high.
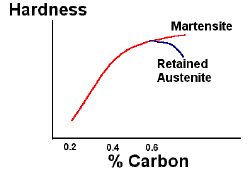
Effect of carbon content (wt%) on hardness
Carbon also increases the hardenability of steels by retarding the formation of pearlite and ferrite. However, the effect is too small be be commonly used for control of hardenability. High carbon steels are prone to distortion and cracking during heat treatment, and can be difficult to machine in the annealed condition before heat treatment. It is more common to control hardenability with other elements, and to use carbon levels of less than 0.4wt%.
Boron
Boron is a very potent alloying element, typically requiring 0.002 to 0.003wt% to have an equivalent effect as 0.5wt% Mo. The effect of boron is also independent of the amount of boron, provided sufficient is added, and the effect of boron is greatest at lower carbon contents. It is typically used with lower carbon steels.
Boron has a very strong affinity for oxygen and nitrogen, with which it forms compounds. Boron can therefore only affect the hardenability of steels if it is in solution. This requires the addition of "gettering" elements such as aluminium and titanium to react preferentially with the oxygen and nitrogen in the steel.
Chromium, molybdenum, manganese, silicon, nickel, vanadium
The elements Cr, Mo, Mn, Si, Ni and V all retard the phase transformation from austenite to ferrite and pearlite. The most commonly used elements are Cr, Mo and Mn.
The retardation is due to the need for redistribution of the alloying elements during the diffusional phase transformation from austenite to ferrite and pearlite. The solubility of the elements varies between the different phases, and the interface between the growing phase cannot move without diffusion of the slowly moving elements. There are quite complex interactions between the different elements, which also affect the temperatures of the phase transformation and the resultant microstructure. Steel compositions are sometimes described in terms of a carbon equivalent which describes the magnitude of the effect of all of the elements on hardenability.
Grain size
Increasing the austenite grain size increases the hardenability of steels. The nucleation of ferrite and pearlite occurs at heterogeneous nucleation sites such as the austenite grain boundaries. Increasing the austenite grain size therefore decreases the available nucleation sites, which retards the rate of the phase transformation. This method of increasing the hardenability is rarely used since substantial increases in hardenability require large austenite grain size, obtained through high austenitisation temperatures. The resultant microstructure is quite coarse, with reduced toughness and ductility.
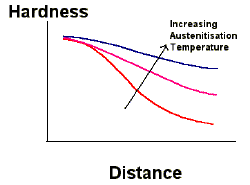
Effect of austenite grain size on hardenability
The austenite grain size can be affected by other stages in the processing of steel, and therefore the hardenability of a steel also depends on the previous stages employed in its production.

