17.10: Questions
- Page ID
- 36620
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Quick questions
You should be able to answer these questions without too much difficulty after studying this TLP. If not, then you should go through it again!
The Reynolds number is a measure of:
- Answer
-
C
The terminal velocity of a particle falling under gravity in a fluid is:
- Answer
-
A
The Stokes number is a measure of:
- Answer
-
D
The (specific) permeability of a porous medium is:
- Answer
-
A
The sintering process that leads to a relatively loose assembly of powder particles being transformed into a material with acceptable strength and stiffness:
- Answer
-
C
Deeper questions
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP.
It is proposed that a new type of Diesel Particulate Filter (DPF), with a very high efficiency for extracting fine carbon particles, could be made by creating a bonded assembly of fine alumina fibres (diameter ~0.1 μm). Use the Carman-Kozeny equation to estimate the expected permeability of such a material, assuming that it will be 80% porous. If the maximum gas flux through the DPF is 500 m3 hr-1, its surface area is ~1 m2 and its wall thickness is ~1 mm, estimate the pressure drop across it and comment on whether this value is likely to be acceptable for satisfactory operation of the engine. [The viscosity of the exhaust gas at the temperature concerned is about 3 10-5 Pa s.]
- Answer
-
All that is required to use the C-K equation is the value of S. For an open assembly of (long) fibres, taking the total volume as V, the porosity as P, the total fibre length as L and the fibre radius as R:
\[V_{fib} = \left( {1 - P} \right)V = L\left( {\pi {R^2}} \right)\]
so the value of S can be expressed as\[S = \frac{L\left( {2\pi R} \right)}{V} = \frac{\left( {1 - P} \right)L\left( {2\pi R} \right)}{L\left( {\pi {R^2}} \right)} = \frac
(click for details){R} = \frac{0.4}{{{10^{ - 7}} = 4\;{10^6}\;{\rm{m}^2}\;{\rm{m}^{-3}}\]Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[1]/dd/p[3]/span, line 1, column 2The permeability is thus given by
\[\kappa = \frac
(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[1]/dd/p[5]/span[1], line 1, column 3(click for details)= \fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[1]/dd/p[5]/span[2], line 1, column 3(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[1]/dd/p[5]/span[3], line 1, column 2(click for details)\approx 2\;{10^{ - 13}}\;{{\rm{m}}^2}\]Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[1]/dd/p[5]/span[4], line 1, column 3Darcy's law can now be used to estimate the required pressure drop
\[\Delta p = \frac{Q\eta \Delta x}{\kappa } = \frac{500\left( 3\; 10^{-5} \right)10^{-3}} {3600\left( 2 \;{10^{-13} \right)} \approx 2\;10^4 \; \rm{Pa} \approx 0.2\;\rm{bar}\]
It is proposed that thermal spraying will be used to create an alumina (MPt. ~2040˚C) coating on a metal artefact, using a combustion torch with a flame temperature of 2300˚C. The alumina powder to be used has an average particle size of about 20 µm and the heat capacity of alumina c, is ~3 106 J m-3 K-1. The stand-off distance to be used is 400 mm and the gas velocity is 200 m s-1. The interfacial heat transfer coefficient under these conditions is estimated to be 10 kW m-2 s-1. Assuming that injected particles reach the gas velocity very quickly, estimate their temperature at impact and hence decide whether the spraying process is likely to be successful.
- Answer
-
The key issue here is the particle temperature at impact, Ti, after a flight time of tf in contact with gas at a temperature Tg (flame temperature). It can be assumed that, during thermal spraying, particles will remain approximately isothermal throughout (Bi<<1). The heating rate can be obtained from the heat flux across the interface, which is dictated by the temperature difference ΔT and the heat transfer coefficient, hi:
\[\begin{array}{l}{h_{\rm{i}}}\Delta T\left( {4\pi
(click for details)\right) = c\left( {\fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[1], line 1, column 1(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[2], line 1, column 2(click for details)} \right)\left( {\frac{4}{3}\piCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[3], line 1, column 2(click for details)\right)\\∴ \fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[4], line 1, column 1(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[5], line 1, column 2(click for details)= \fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[6], line 1, column 2(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[7], line 1, column 5(click for details)\end{array}\]Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[2]/span[8], line 1, column 1The heating rate thus drops during flight, as the temperature difference across the interface becomes smaller. The above equation can be used to relate the flight time to the corresponding temperature change of the particle (from T0 to Ti), in integral form:
\[{t_{\rm{f}}} = \int\limits_0^
(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[1], line 1, column 4(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[2], line 1, column 1(click for details)\int\limits_Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[3], line 1, column 5(click for details)^Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[4], line 1, column 1(click for details){\fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[5], line 1, column 4(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[6], line 1, column 2(click for details)} \]Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[4]/span[7], line 1, column 1Carrying out the integration:
\[\begin{array}{l}{t_{\rm{f}}} = \frac(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[1], line 1, column 1(click for details)\left[ {\ln \left( {\fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[2], line 1, column 5(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[3], line 1, column 4(click for details)} \right)} \right]\\∴ {T_{\rm{i}}} = {T_{\rm{g}}} - \left(Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[4], line 1, column 4(click for details)\right)\exp \left( {\fracCallstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[5], line 1, column 4(click for details)Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[6], line 1, column 8(click for details)} \right)\end{array}\]Callstack: at (Bookshelves/Materials_Science/TLP_Library_II/17:_Powder_Processing/17.a10:_Questions), /content/body/div[2]/dl[2]/dd/p[5]/span[7], line 1, column 1Since it is being assumed that the particle instantly reaches the gas velocity, u (= 200 m s-1), and the flight distance is 400 mm, the flight time is 2 ms. The particle temperature at impact is therefore given by:
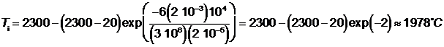
This is below the melting point and so there would be a concern about the particles being sufficiently soft. (In fact, in order to melt the particles, it would also be necessary to inject the required latent heat, so they would be some way from being molten.) The spraying is therefore not expected to be successful.


