5.2: Enthalpy
- Page ID
- 88846
Enthalpy is an important thermodynamic property for the analysis of energy conservation in open systems. It combines the internal energy and flow work associated with the flowing fluid (see Section 2.2.5 for details). The following sections explain how to determine the specific enthalpy at a given state.
5.1.1 Using thermodynamic tables to determine specific enthalpy h
As described in Chapter 2, thermodynamic tables can be used to determine thermodynamic properties, such as pressure, temperature, specific volume, specific internal energy, specific enthalpy, and specific entropy of a pure substance at a given condition. After the specific enthalpy is found, the enthalpy can then be calculated by using the following equation:
\[H = mh\]
where
\[H\]
\[h\]
\[m\]
Example 1
Find the missing properties of R134a and ammonia at the given conditions.
| Substance | T, oC | P, kPa | h, kJ/kg | x | Phase | |
| 1 | R134a | 20 | 380 | |||
| 2 | Ammonia | -20 | 200 |
Solution
1. R134a at T = 20oC has a specific enthalpy of h = 380 kJ/kg

2. Ammonia at a temperature of T = -20oC and a pressure of P = 200 kPa
In summary,
| Substance | T, oC | P, kPa | h, kJ/kg | x | Phase | |
| 1 | R134a | 20 | 571.71 | 380 | 0.83679 | Two-phase mixture |
| 2 | Ammonia | -20 | 200 | 251.71 | n.a. | Compressed liquid |
5.1.2 Constant-pressure specific heat
Constant-pressure specific heat is defined as the energy required to raise the temperature of a unit mass (i.e., 1 kg) of a substance by 1 degree (i.e., 1oC, or 1 K) in an isobaric process. Mathematically, it is expressed as
\[C_p=\left(\displaystyle\frac{\partial\ h}{\partial\ T}\right)_p\]
where
\[C_{p}\]
\[h\]
\[T\]
The constant-pressure specific heat of selected substances can be found in Appendix G. For example, the constant-pressure specific heat of air at 300 K is 1.005 kJ/kgK, see Table G1. Let us consider one kilogram of air originally at 300 K in a piston-cylinder device. It will require 1.005 kJ of heat for the air temperature to increase from 300 K to 301 K if the piston-cylinder device is heated to allow the air to expand in an isobaric process.
It is important to note that both specific heats, 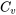 and
and 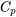 , are properties of a substance. Although they are typically measured in isochoric and isobaric processes, respectively, their applications are NOT limited to isochoric or isobaric processes. For ideal gases,
, are properties of a substance. Although they are typically measured in isochoric and isobaric processes, respectively, their applications are NOT limited to isochoric or isobaric processes. For ideal gases, 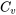 and
and 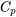 can be used to determine
can be used to determine 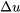 and
and 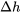 , respectively, in ANY processes.
, respectively, in ANY processes.
5.1.3 Using Cp to calculate Δh for ideal gases
The specific enthalpy of an ideal gas is a function of temperature only, 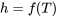 ; therefore,
; therefore,
\[C_p=\left(\displaystyle\frac{\partial\ h}{\partial\ T}\right)_p = \left(\dfrac{dh}{dT}\right)_p=f(T)\]
The change of specific enthalpy of an ideal gas within a small temperature range can be calculated as
\[\Delta h = h_2-h_1 = C_p(T_2-T_1)\]
where
\[C_{p}\]
\[h\]
\[T\]
Subscripts 1 and 2 represent states 1 and 2 in a process, respectively.
The above formula is a simple, approximate method to estimate the change of specific enthalpy of an ideal gas due to temperature variations. It is reasonably accurate and may be used when the thermodynamic tables of an ideal gas are not available. Table G1 lists the constant-pressure specific heat of selected substances at 300 K. Strictly speaking, since 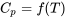 ,
, 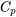 at a different temperature should be calculated according to that specific temperature. However, Table G1 is often used in approximate calculations as long as the temperature variations of the ideal gas remain in a small range.
at a different temperature should be calculated according to that specific temperature. However, Table G1 is often used in approximate calculations as long as the temperature variations of the ideal gas remain in a small range.
The specific heat ratio, 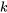 , of an ideal gas is defined as the ratio of
, of an ideal gas is defined as the ratio of 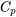 to
to 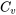 .
.
\[k=\displaystyle\frac{C_p}{C_v}\]
The following equations relate 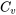 ,
, 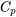 ,
, 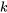 , and
, and 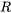 . Detailed derivations are omitted here.
. Detailed derivations are omitted here.
\[C_p=C_v+R\]
5.1.4 Using Cp to calculate Δh for solids and liquids
Liquids and solids are generally treated as incompressible substances because their volumes do not change with pressure or temperature significantly. For liquids and solids, the difference between the constant-volume specific heat and the constant-pressure specific heat is typically negligible; therefore,
\[C_p \approx C_v\]
\[\Delta h \approx\ \Delta u\approx\ C_p(T_2-T_1)\]
Example 2
Consider a piston-cylinder device containing 2 kg of oxygen at 300 K, 200 kPa. The piston-cylinder device is made of 0.5 kg of aluminum. How much heat is required for the oxygen to reach a temperature of 600 K in an isobaric process? Assume the oxygen and the piston-cylinder are always in thermal equilibrium during the isobaric process. The oxygen can be treated as an ideal gas in this heating process.
Solution
First, set up a closed system as shown outlined in blue, which consists of the piston-cylinder and O2.
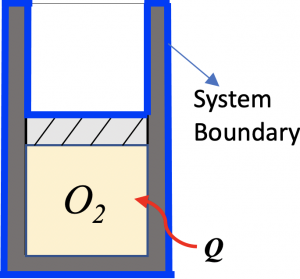
Second, apply the first law of thermodynamics to the closed system. Note that, in the heating process, the temperatures and internal energies of both O2 and the piston-cylinder increase.
\[\because \Delta U_{tot} = Q_{tot} - W_{O_{2}}\]
\[\therefore Q_{tot} = \Delta U_{tot} + W_{O_{2}} = \Delta U_{al} + (\Delta U_{O_{2}} + W_{O_{2}})\]
Third, calculate the change in internal energy of the piston-cylinder. From Table G3, the constant-pressure specific heat for aluminum is 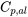 = 0.897 kJ/kgK.
= 0.897 kJ/kgK.
\[\begin{align*} \Delta U_{al} &= m_{al}\Delta u_{al} = m_{al}\Delta h_{al} \\&= m_{al}C_{p,al}(T_2 - T_1) \\&= 0.5 \times 0.897 \times (600-300)= 134.55 \ \rm{kJ}\end{align*}\]
Fourth, analyze the boundary work done by O2 and the change in internal energy of O2 in this isobaric process, where  .
.
\[\begin{align*} \because \; \; W_{O_{2}} &= P(\mathbb{V}_2 - \mathbb{V}_1) \; \; \; \; \Delta U_{O_{2}} = U_2 - U_1 \\ H &=U+P\mathbb{V} \end{align*}\]
\[\begin{align*} \therefore \; \Delta U_{O_{2}} + W_{O_{2}} &= (U_2 - U_1) + P(\mathbb{V}_2 - \mathbb{V}_1) \\&= (U_2 + P_2\mathbb{V}_2) - (U_1 + P_1\mathbb{V}_1) \\&= H_2 - H_1 \end{align*}\]
\[\therefore \Delta U_{O_{2}} + W_{O_{2}} = \Delta H_{O_{2}}\]
From Table G1, the constant-pressure specific heat for oxygen is 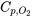 = 0.918 kJ/kgK.
= 0.918 kJ/kgK.
\[\begin{align*} \therefore \Delta H_{O_{2}} &= m_{O_{2}}C_{p,O_{2}}(T_2 - T_1) \\&= 2 \times 0.918 \times (600-300) = 550.8 \ \rm{kJ} \end{align*}\]
Last, calculate the total heat transfer in this process.
\[\begin{align*} Q_{tot} &= \Delta U_{al} + \Delta H_{O_{2}} \\&= 134.55+550.8= 685.35 \ \rm{kJ} \end{align*}\]
Query \(\PageIndex{1}\)


