6.10: The second law of thermodynamics for open systems
- Page ID
- 88860
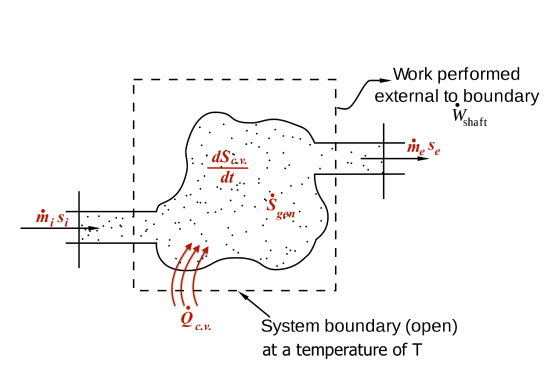
Entropy can be transferred to a system via two mechanisms: (1) heat transfer and (2) mass transfer. For open systems, the second law of thermodynamics is often written in the rate form; therefore, we are interested in the time rate of entropy transfer due to heat transfer and mass transfer.
\[\dot{S}_{heat} =\dfrac{dS_{heat}}{dt} \cong \displaystyle\sum\dfrac{\dot{Q}_k}{T_k}\]
\[\dot{S}_{mass} = \displaystyle\sum\dfrac{dS_{mass}}{dt} = \displaystyle \sum \dot{m}_{k}s_{k}\]
where
\[\dot{m}\]
\[\dot{Q}_k\]
\[\dot{S}_{heat}\]
\[\dot{S}_{mass}\]
\[s_k\]
Applying the entropy balance equation, 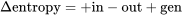 , to a control volume, see Figure 6.9.1, we can write the following equations:
, to a control volume, see Figure 6.9.1, we can write the following equations:
- General equation for both steady and transient flow devices
\[\dfrac
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[1], line 1, column 4
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[2], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[3], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[4], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[5], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[6], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[7], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[8], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[9], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[1]/span[10], line 1, column 1
- For steady-state, steady-flow devices,
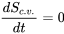 ; therefore,
; therefore,
\[\displaystyle \sum
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[1], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[2], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[3], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[4], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[5], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[6], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[7], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[1]/span[8], line 1, column 1
- For steady and isentropic flow devices,
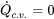 and
and 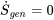 ; therefore,
; therefore,
\[\displaystyle\sum
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[2]/span[1], line 1, column 1
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/div[4]/p[2]/span[2], line 1, column 1
where
\[\dot{m}\]
\[\dot{Q}_{c.v.}\]
\[S_{c.v.}\]
\[\dfrac
Callstack:
at (Bookshelves/Mechanical_Engineering/Introduction_to_Engineering_Thermodynamics_(Yan)/06:_Entropy_and_the_Second_Law_of_Thermodynamics/6.10:_The_second_law_of_thermodynamics_for_open_systems), /content/body/p[6]/span, line 1, column 3
\[\dot S_{gen}\]
\[s\]
\[T\]
Example 1
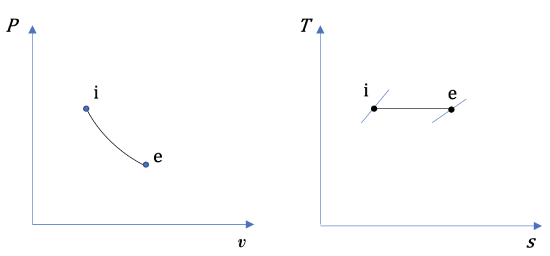
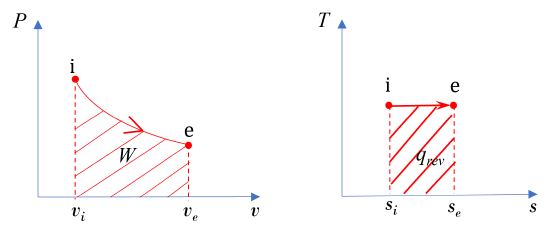
The diagrams in Figure 6.9.e1 show a reversible process in a steady-state, single flow of air. The letters i and e represent the initial and final states, respectively. Treat air as an ideal gas and assume ΔKE=ΔPE=0. Are the change in specific enthalpy Δh=he−hi, specific work w, and specific heat transferq positive, zero, or negative values? What is the relation between w and q?
Solution:
The specific work can be evaluated mathematically and graphically.
(1) Mathematically,
\[\because v_{e} > v_{i}\]
\[\therefore w = \displaystyle\int_{i}^{e}{Pd{v}\ } >\ 0\]
(2) Graphically, the specific work is the area under the process curve in the 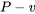 diagram; therefore
diagram; therefore 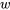 is positive, see Figure 6.9.e2.
is positive, see Figure 6.9.e2.
In a similar fashion, the specific heat transfer can also be evaluated graphically and mathematically.
(1) Graphically,
\[\because ds=\left(\displaystyle\frac{\delta q}{T}\right)_{rev}\]
\[\therefore q_{rev} = \displaystyle \int_{i}^{e}{Tds} = T(s_e-s_i)\ >\ 0\]
For a reversible process, the area under the process curve in the 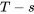 diagram represents the specific heat transfer of the reversible process; therefore
diagram represents the specific heat transfer of the reversible process; therefore 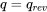 is positive, see Figure 6.9.e2.
is positive, see Figure 6.9.e2.
(2) The same conclusion, 0" class="latex mathjax" title="q_{rev}>0" src="/@api/deki/files/59236/d00f283ba44c47860e35c0b010cd6fb7.png">, can also be derived from the second law of thermodynamics mathematically, as follows.
\[\dot{m}(s_e-s_i)=\displaystyle\sum\frac{\dot{Q}}{T_{surr}}+\dot{S}_{gen}\]
For a reversible process, 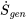 = 0, and the fluid is assumed to be always in thermal equilibrium with the system boundary, or
= 0, and the fluid is assumed to be always in thermal equilibrium with the system boundary, or 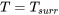 ; therefore,
; therefore,
\[q_{rev} = \dfrac{\dot{Q}}{\dot{m}} = T(s_e-s_i) > 0\]
The change in specific enthalpy can then be evaluated. For an ideal gas,
\[\Delta h = h_e - h_i = C_p(T_e - T_i)\]
\[\because T_e = T_i\]
\[\therefore h_e = h_i\]
Now, we can determine the relation between 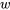 and
and 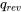 from the first law of thermodynamics for control volumes.
from the first law of thermodynamics for control volumes.
\[\because \dot{m}( h_e - h_i ) = \dot{Q}_{rev} - \dot{W} = 0\]
\[\therefore \dot{Q}_{rev} = \dot{W}\]
\[\therefore q_{rev} = w\]
In this reversible process, the specific heat transfer and specific work must be the same. Graphically, the two areas under the 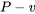 and
and 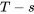 diagrams must be the same.
diagrams must be the same.
Query \(\PageIndex{1}\)
Media Attributions
- Entropy transfers and entropy generation through a C.V. © Pbroks13 is licensed under a Public Domain license


