4.2: Nuclear Physics- Brief Review of Fundamental Facts
- Page ID
- 84595
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have to start with a brief review of fundamental facts in nuclear physics. So, atoms consist of “shells” of electrons, which surround a nucleus a tiny col- lection of particles known as “nucleons”: there are two kinds of them, namely, protons (positively charged particles, with a charge of the same magnitude as the negative electron charge) and neutrons (particles only slightly more massive then protons, but with no electric charge). The size of an atom is of the order of 10−10 m – one tenth of one billionth of a meter. The size of a nucleus is about one hundred thousand times smaller than the size of an atom.
So, in summary:
- An atom consists of a positive nucleus, and a “shell” of negative elec- trons;
- A nucleus consists of positive protons and neutrons that have no charge. The proton and electron charges are of the same magnitude, but of opposite signs;
- The number of protons in the nucleus is the same as the number of electrons in the electronic shell, so that the atom has a zero net charge.
- There is one common name for the protons and neutrons comprising an atomic nucleus – the nucleons. As, for example, in a kindergarten class: one may say: There are 10 girls and 8 boys in the class, or: There are 18 children in the class.
Particles with positive electric charge repel one another, and protons in the nucleus are so closely packed that these repulsive forces are very strong. So, in atomic nuclei there is an extra attractive force that holds all the constituent protons and neutrons together. It’s known as the “strong force”. It would be too long a story to talk about this force in our course but if you are interested, much material about strong nuclear forces can be found in the Web, e.g., in this Wikipedia article. The only important fact that we need to point out here is that neutrons play an important role in holding the nucleus together. With the exception of the hydrogen nucleus, consisting of a single proton, there are no other nuclei with no neutrons. And the number of neutrons cannot be too low or too high. The rules are not simple, it’s better to use a graph to explain what is going on.
Figure \(\PageIndex{1}\) shows the so-called “Chart of known nuclei”. There are stable nuclei, and radioactive (unstable) nuclei, that decay after some time. The “lifetimes” of known unstable nuclei span from microseconds to billions of years.
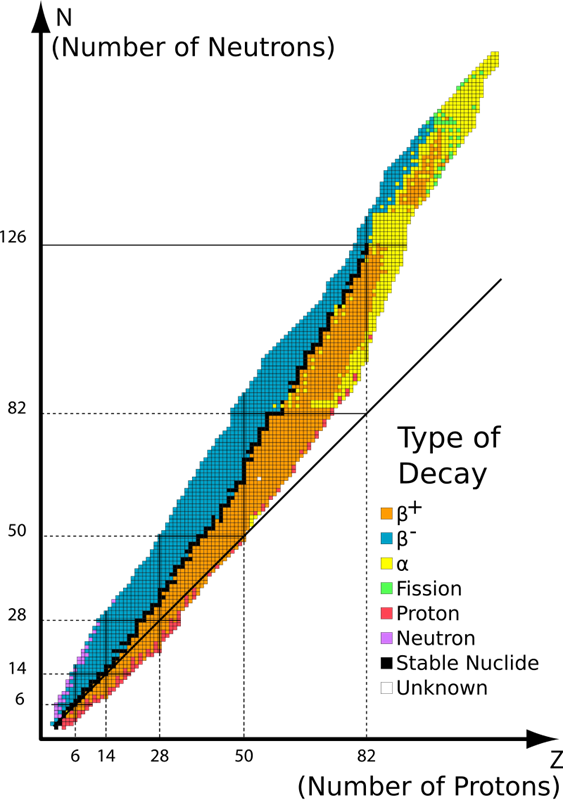
The stable nuclei – we know 253 of them – form the so-called “stability path” (black squares in the graph). As you can see, the ratio of the number of protons (Z ) to the number of neutrons (N ) is about 1 for the light stable nuclei, and about 2/3 for the heavy ones. There are over 5000 known radioactive (unstable) nuclei, about 350 of them occurring naturally, and the rest produced artificially, most often by bombarding stable nuclei with high-energy protons or ions.
The graph shows the properties of nuclei depending on the number of constituent neutrons (on the abscissa) and protons (on the ordinate). Black dots indicate stable nuclei, blue dots unstable nuclei, i.e. such that have a limited lifetime only and eventually undergo a radioactive decay.
Notation: By convention, Z is the number of protons in a nucleus. Because each proton has a positive electric charge of the same magnitude as the negative charge of an electron, Z can also be thought of as the total charge of a given nucleus expressed in the units of electron charge. N is the number of neutrons, and A is the total number of all nucleons therefore, A = Z + N . Also, the convention is that for describing nuclei we use the chemical symbol and put Z as the left subscript and A as the left superscript: AX. One small comment: Z, the number of protons in the nucleus is equal to the number of electrons in a given atom, because atoms are electrically neutral. So, in fact, writing Z in the nucleus’ symbol is redundant. Therefore, Z is often omitted for instance, instead of 12C, we more often write simply 12C. Another small comment: sometimes people instead of using the left sub- and super- scripts (its the official way, sanctioned by international organizations) put Z and A as the right sub- and superscripts, e.g., C12, or simply C12. Such “unorthodox” notation seldom leads to misunderstandings, though. By the way, there is an often-used alternative way of describing nuclei – one uses not the chemical symbol, but the full name of the element, starting with a capital, and then a dash and the A number: e.g., Carbon-12, Carbon-14, or Uranium-235.
The subscript + superscript convention is also used for single particles. The proton and the neutron are both nucleons, so for both A = 1. For protons Z = 1, and for neutral neutrons Z = 0, so for them we use the symbols:
\[{ }_{1}^{1} p \text { and }{ }_{0}^{1} n \]
respectively. The electron is not a nucleon, but since electrons take part in several types of nuclear processes, one can use the same notation for them, with \(A=\) o (meaning: "not a nucleon"), and \(Z=-1\) :
\[{ }_{-1}^{\;\;0} e \text { or } { }_{-1}^{\;\;0} \beta \]
are two equivalent symbols. Finally, there are two more particle types created in nuclear processes: gamma radiation quantum, and neutrinos. Neither is a nucleus nor has is an electric charge, so their symbols are:
\[{ }_{0}^{0} \gamma \text{ and } { }_{0}^{0} \nu\],
respectively.
Note that the number of neutrons in stable nuclei is either equal to the number of protons ( \(N=Z\), as in some light nuclei, such as, e.g., in carbon \({ }_{6}^{12} \mathrm{C}\) ), or greater , \(N>Z\). There is only one known exception from this rule, a stable nucleus with two protons and only one neutron: \(Z=2\) identifies it as helium, so that the full symbol is \({ }_{2}^{3} \mathrm{He}\) (it's an extremely rare isotope of helium, in ordinary helium nucleus there are two neutrons). For heavier nuclei, the number of neutrons grows faster, than the number of protons. In the heaviest of all stable nuclei, an isotope of lead, \(Z=82\) and \(A=208\) \(\left({ }_{82}^{208} \mathrm{~Pb}\right)\), so that he \(N / Z\) ratio in it is \((208-82) / 82=126 / 82=1.54\).
Isotopes: Atoms with nuclei in which the number of protons \((Z)\) is the same, but with different numbers of neutrons, are called isotopes of the same chemical element. The chemical properties of atoms depend on the number of electrons in their shells or, equivalently, on the number of protons \(Z\) in their nuclei. Atoms with different number of electrons are different chemical elements:
\[ \begin{array}{l}
Z=1: \text { Hydrogen; } \\
Z=2: \text { Helium; } \\
Z=3: \text { Lithium; } \\
Z=4 \text { : Beryllium; } \\
Z=5: \text { Boron; } \\
Z=6: \text { Carbon; } \\
. . . . . . . . . . \\
. . . . . . . . . . \\
Z=92: \text{ Uranium}
\end{array} \]
However, there may be several nuclei with the same number of protons \(Z\), but a different number of neutrons \(N\). Atoms with such nuclei have all the same number of electrons, which determines their chemical properties. They are all atoms of the same chemical element, but they are different isotopes of that element. Some even have special names: \(Z=1, N=0\) is the "ordinary" Hydrogen; \(Z=1, N=1\) is Deuterium (stable); \(Z=1, N=3\) is Tritium (radioactive). But for \(Z\) greater or equal 2 different names are no longer used. Instead, they have the same name and are distinguished by the mass number \(A\) - e.g.: Carbon-12, Carbon-13, and carbon-14; or Uranium-233, Uranium-235, and Uranium-238.

