8.1: Review of Refinery Processing and Chemical Structures for Jet Fuel and Diesel Fuel
- Page ID
- 48592
8.1 Review of Refinery Processing and Chemical Structures for Jet Fuel and Diesel Fuel
Recall from Lesson 2 the general schematic of a refinery, shown here in Figure 8.1. Jet fuel is typically in the middle distillate range, also known as naphtha and kerosene. Diesel fuel is heavier (higher molecular weight and longer long-chain hydrocarbons). These fuels do not require as much processing because they can be obtained primarily from distillation of oil, but because of sulfur/oxygen/nitrogen functional groups and high molecular weight waxes, these fuels must have these components removed. They are hydrotreated (hydrogen is added, sulfur/oxygen/nitrogen are removed, and aromatics are made into cycloalkanes). Waxes are also separated out.

- Click here for a text alternative of Figure 8.1
-
This is a simple flow diagram of crude oil refinery.
Crude oil enters and goes to distillation.
From distillation:
LPG (gases) goes through alkylation to become O.N. 100 Motor Fuel Alkylate which can go one to become gasoline
Straight-run gasoline goes through catalytic reforming to become O.N. 95 Reformate which can go on to become gasoline
Naphtha, Kerosene and Diesel all go through Hydrotreating and then dewaxing to become either treated Kerosene, Diesel (low sulfur) or lubricating oils.
Fuel Oil goes through a catalytic cracker to become O/N 90-95 Gasoline
Resid goes through Thermal Cracking to become either Carbon, Asphalt or O.N. 75 Gasoline.
Credit: Dr. Caroline B. Clifford
The primary structure we want for jet fuel and diesel fuel is:
Alkane - atoms are lined up. For stick representation, each corner represents a CH2 group, and each end represents a CH3 group.
| Name | Atoms and Bonds | Stick Representation |
|---|---|---|
| Heptane (7 C atoms) | 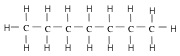 |
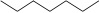 |
Cycloalkanes - again, still an alkane, but forms a ring compound.
| Name | Atoms and Bonds | Stick Representation |
|---|---|---|
| Cyclohexane (6 C atoms) | 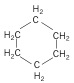 |
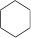 |
Table 8.1 also shows a list of different chemicals and the properties of each. This table is mainly focused on those chemicals that would be in jet and diesel fuels.
Table 8.1: List of common hydrocarbons and properties
| Name | Number of C Atoms | Molecular formula | bp (0C), 1 atm | mp (0C) | Density (g/mL)(@200C) |
|---|---|---|---|---|---|
| Decane | 10 | C10H22 | 174.1 | -30 | 0.760 |
| Tetradecane | 14 | C14H30 | 253.5 | 6 | 0.763 |
| Hexadecane | 16 | C16H34 | 287 | 18 | 0.770 |
| Heptadecane | 17 | C17H36 | 303 | 22 | 0.778 |
| Eicosane | 20 | C20H42 | 343 | 36.8 | 0.789 |
| Cyclohexane | 6 | C6H12 | 81 | 6.5 | 0.779 |
| Cyclopentane | 5 | C5H10 | 49 | -94 | 0.751 |
| Benzene | 6 | C6H6 | 80.1 | 5.5 | 0.877 |
| Naphthalene | 10 | C10H8 | 218 | 80 | 1.140 |
| Tetrahydronaphthalene(tetralin) | 10 | C10H12 | 207 | -35.8 | 0.970 |
| Decahydronaphthalene(decalin) | 10 | C10H18 | 187,196 | -30.4, -42.9 | 0.896 |


