2.3: Reaction Order
- Page ID
- 101147
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By the end of this section, you should be able to:
- Define reaction order and overall order
Determine the reaction order and the rate constant from kinetic data
The dependence of the rate of reaction on the reactant concentrations can often be expressed as a direct proportionality, in which the concentrations may be raised to be the zeroth, first, or second power. The exponent is known as the order of the reaction with respect to that substance.\(^{[1]}\)
The overall order of a reaction is the sum of the orders with respect to the sum of the exponents
For example:
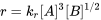
The reaction is:
- Third-order in A
- One-half order in B
- Three and a half order overall
In the more complicated example for the reaction previously given between hydrogen and bromine:
The reaction is:
- First order in \(H_{2}\)
- Indeterminate order in \(Br_{2}\) (as it’s not in a single term)
- Indeterminate order in \(H\!Br\) (as it cannot be isolated to a single term raised to a power)
- Indeterminate order overall (as some orders are indeterminate)
Determining the Rate Law
Isolation Method for Determining the Rate Law
One of the simplest methods for determining the rate law is the isolation method.
Say we have a reaction with two reactants, A and B. We put a large amount of B in our reactor compared to A; so much that the concentration barely changes.
If the true rate law is: \(r = k_{r}[A][B]^2\)
What we would observe is \(r = k_{eff}[A]\), where \(k_{eff}=k_{r}[B_{0}]^2\)
- \(k_{eff}\) = the effective rate constant
- \([B]_{0}\)indicating the concentration of B at the start of the reaction (or time zero) or also called B naught (with naught meaning nothing or zero in this case)

With B in great excess: \(r = k_{eff}[A]\) where \(k_{eff}=k_{r}[B_{0}]^2\), reaction appears first order.
With A in great excess: \(r' = k'_{eff}[B]^2\) where \(k'_{eff}=k_{r}[A_{0}]\), reaction appears second order.
\[k'_{eff}\]
It is easier to analyse these individual effective rate laws than the more complex combined rate law when both concentrations change significantly.
Method of Initial Rates
The method of initial rates is commonly used in conjunction with the isolation methods to determine reaction order.
Say we have a reaction with two species, A and B. We put in the same amount of B into the reactor each time. The following is our initial rates are observed with different concentrations of A. What is the reaction order in A?
| [A] (mol/L) | Initial rate(mol/L·s) |
| 1 | \(1×10^{-2}\) |
| 2 | \(4×10^{-2}\) |
Solution
Add example text here.
As the concentration of A doubles, the reaction rate quadruples
\[r_{0}=k_{eff}[A]^2\]
So the reaction is second order in A.
If we take the logarithm of the general equation for reaction rate, we can linearize the equation:
\begin{align*}
r_{0} & = k_{eff}[A_{0}]^a \\
log(r_{0})& = log(k_{eff}) + a*log([A_{0}])\\
y & = intercept + slope * x
\end{align*}
Plot \(log(r_{0})\) vs \(log([A_{0}])\), where the slope is the reaction order in A; and y-intercept equals to \(log(k_{eff})\)
Sulfuryl chloride (\(SO_{2}Cl_{2}\)) decomposes to \(SO_{2}\) and \(Cl_{2}\) by the following reaction:
\[SO_{2}Cl_{2(g)} → SO_{2(g)}+Cl_{2(g)}\]
\[^{[2]}\]
| Experiment | \([SO_{2}Cl_{2}]_{0}\) | Initial rate(M/s) |
| 1 | 0.0050 | \(1.10×10^{-7}\) |
| 2 | 0.0075 | \(1.65×10^{-7}\) |
| 3 | 0.0100 | \(2.20×10^{-7}\) |
| 4 | 0.0125 | \(2.75×10^{-7}\) |
Solution
Add example text here.
Step 1: determine the reaction order with respect to sulfuryl chloride
\[(2.20×10^{-7}) ÷ (1.10×10^{-7}) = 2.0\]
\[[SO_{2}Cl_{2}]\]
\[k_{r}\]
We have \(rate = k_{r}[SO_{2}Cl_{2}]\). We can calculate the rate constant (\(k_{r}\)) using data from any experiment in the table.
\begin{align*}
rate & = k_{r}[SO_{2}Cl_{2}] \\
1.10×10^{-7} M/s& = k_{r}×0.0050M \\
2.2×10^{-5}s^{-1} & = k_{r}
\end{align*}
Method 2
\[log(r_{0})\]

\[log(k_{r})=-4.6576\]
\[SO_{2}Cl_{2}\]
References
[1] Chemistry LibreTexts. 2020. The Rate Law. [online] Available at: <https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/Rate_Laws/The_Rate_Law> [Accessed 23 April 2020].
[2] Chemistry LibreTexts. 2020. 14.4: The Change Of Concentration With Time (Integrated Rate Laws). [online] Available at: <https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/14%3A_Chemical_Kinetics/14.4%3A_The_Change_of_Concentration_with_Time_(Integrated_Rate_Laws)> [Accessed 23 April 2020].

