2.8: Steady-State Approximation
- Page ID
- 101152
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By the end of this section, you should be able to:
- Select the rate-determining step given the consecutive reactions and overall rate law of the reaction
Derive the rate law of a reaction given the consecutive reactions
Derive the rate law under pre-equilibria conditions
\[A\xrightarrow{\text{ka}}I\xrightarrow{\text{kb}}P\]
This approximation assumes intermediate, I, is in a low constant concentration,ie:
\[\frac{d[I]}{dt}=0\]
Generally the approximation is more accurate when \(k_{b}>>k_{a}\) (\(k_{b}\) is much greater than \(k_{a}\)). The bigger the difference, the more accurate the assumption.
\[k_{b}\]
As we saw before with first order reactions: \(\frac{d[A]}{dt}=-k_{a}[A]\) leads to \([A]=[A]_{0}e^{-k_{a}t}\)
Now: \(\frac{d[I]}{dt}=k_{a}[A]-k_{b}[I]≈0\) so \([I]=\frac{k_{a}}{k_{b}}[A]\)
For product: \(\frac{d[P]}{dt}=k_{b}[I]≈k_{a}[A]\) if we solved this, we would find: \([P]=[A]_{0}(1-e^{-k_{a}t})\)
Detailed proof: This is not required for the course, but might be useful for understanding.
For reactant:
\[\frac{d[A]}{dt}=-k_{a}[A]\]
\[[A]\]
\[[A]=constant*e^{-k_{a}t}\]
\[[A]=[A]_{0}*e^{-k_{a}t}\]
For intermediate:
\[\frac{d[I]}{dt}=k_{a}[A]-k_{b}[I]≈0\]
For product:
\[\frac{d[P]}{dt}=k_{b}[I]≈k_{a}[A]\]
\begin{align*}
\frac{d[P]}{dt} & = k_{a}[A] \\
\frac{d[P]}{dt} & = k_{a}*[A]_{0}*e^{-k_{a}t}\\
[P] & = \int k_{a}*[A]_{0}*e^{-k_{a}t}\\
[P] & = -[A]_{0}*e^{-k_{a}t} + constant
\end{align*}
To find the constant, we know that there is no product in the system, initially. At t=0, [P]=0. Subsitute t=0, the exponential term is equal to 1, so we are left with \(0=-[A]_{0}+constant\), so \(constant=[A]_{0}\), Therefore:
\[[P]= -[A]_{0}*e^{-k_{a}t} + [A]_{0}=[A]_{0}(1-e^{-k_{a}t})\]
Taking arbitrary values of \(k_{a}=1\) and \(k_{b}=10\), we can plot the concentration vs. time for all the components:

To derive the overall rate law for this reaction:
\[2N_{2}O_{5(g)}→4NO_{2(g)}+O_{2(g)}\]
Assume the reaction follows the following three-step mechanism:
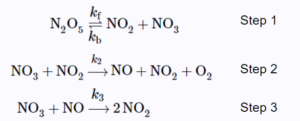
The final expression of the rate law should only contain \([N_{2}O_{5}]\) and the rate constants of the elementary steps.\(^{[1]}\)
Solution
Add example text here.
Step 1: Find the intermediates and express their rate of formation. Equate their rate of formation to 0 due to steady-state approximation.
\[\frac{d[NO]}{dt}=k_{2}[NO_{3}][NO_{2}]-k_{3}[NO_{3}][NO]=0\;\;\;\;\;(1)\]
\[\frac{d[NO_{3}]}{dt}=k_{f}[N_{2}O_{5}]-k_{b}[NO_{2}][NO_{3}]-k_{2}[NO_{3}][NO_{2}]-k_{3}[NO_{3}][NO]=0\;\;\;\;\;(2)\]
Step 2: Express the overall rate law. The easiest way is to express \(\frac{d[O_{2}]}{dt}\) because it is a product that has stoichiometric coefficient of 1.
\[\frac{d[O_{2}]}{dt}=k_{2}[NO_{3}][NO_{2}]\]
Step 3: From the expression of \(\frac{d[O_{2}]}{dt}\), we can see that to find the overall rate law, we need to find \([NO_{3}][NO_{2}]\) as an expression of the concentrations of reactants or products (no intermediates) by manipulating (1) and (2).
From (1):
\[k_{2}[NO_{3}][NO_{2}]=k_{3}[NO_{3}][NO]\]
Substitute into (2):
\begin{align*}
k_{f}[N_{2}O_{5}]-k_{b}[NO_{2}][NO_{3}]-k_{2}[NO_{3}][NO_{2}]-k_{2}[NO_{3}][NO_{2}] & = 0\\
k_{b}[NO_{2}][NO_{3}]+2k_{2}[NO_{3}][NO_{2}] & = k_{f}[N_{2}O_{5}]\\
[NO_{3}][NO_{2}] & =\frac{k_{f}[N_{2}O_{5}]}{k_{b}+2k_{2}}
\end{align*}
Step 4: We can substitute the expression for \([NO_{3}][NO_{2}]\) we just found into the expression for \(\frac{d[O_{2}]}{dt}\), which is the same as the overall rate law.
\[r=\frac{d[O_{2}]}{dt}=\frac{k_{2}k_{f}[N_{2}O_{5}]}{k_{b}+2k_{2}}\]
Rate-Determining Step
Rate determining step is the step that determines the overall rate of reaction in a series of reactions. The slowest forward reaction step is referred to as the rate-determining step, as it limits the rate of the entire reaction. Note that this must be a forward reaction step, a reverse reaction in an equilibrium reaction cannot be the rate-determining step.
An analogy that illustrates this concept is an hourglass having two different sized openings. The rate of the sand falling to the bottom-most chamber is determined by the smaller of the two openings. Similarly, the rate law of the overall reaction is determined from its rate-determining slowest step.\(^{[2]}\)
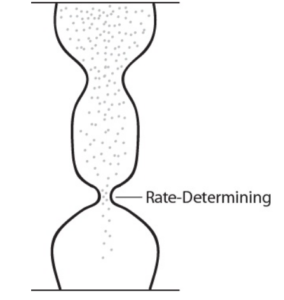
Image from Introduction to Chemistry, 1st Canadian Edition / CC BY 4.0
Pre-Equilibria
Say we have the following forward and reverse reaction:
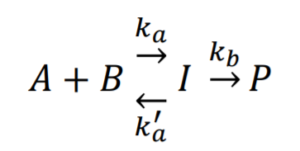
Consider a case where an equilibrium is established due to \(k_{a}’ >> k_{b}\):
The equilibrium constant, expressed using the forward and reverse reaction constants (\(k_{a}\) and \(k_{a}’\)) would remain the same over time
The reaction of \(I→P\) would decrease \([I]\) over time. Due to Le Chatelier’s principle, the equilibrium reaction will shift to produce more \(I\)
Because \(k_{a}'>>k_{b}\), the reaction will shift concentrations to achieve equilibrium again far faster than the forward reaction (\(k_{b}\)) depletes \(I\)
Therefore, we can approximate \([I]\) with only the equilibrium part of the reaction
| \(K=\Big(\frac{[I]}{[A][B]}\Big)_{eq}c^\theta=\frac{k_{a}}{k'_{a}}c^\theta\) |
Review: From lecture 4: reaction equilibrium:
The forward reaction: \(A+B\xrightarrow{\text{ka}}I\), reaction rate \(r=k_{a}[A][B]\)
The reverse reaction: \(I\xrightarrow{\text{k'a}}A+B\), reaction rate \(r'=k_{a}'[I]\)
At equilibrium: \begin{align*}
r & =r’\\
k_{a}[A][B] & = k_{a}'[I]\\
\frac{k_{a}}{k_{a}’}& = \frac{[I]}{[A][B]}
\end{align*}
So
| \([I]=\frac{K}{c^\theta}[A][B]=\frac{k_{a}}{k_{a}'}[A][B]\) |
Using the equation for \([I]\) based on this equilibrium, we can develop an equation for the rate of formation of \(P\):
| \(\frac{d[P]}{dt}=k_{b}[I]=k_{b}\frac{k_{a}}{k_{a}'}[A][B]=k_{r}[A][B]\) |
Where \(k_{r}=k_{b}\frac{k_{a}}{k_{a}'}\). The final step of \(I→P\) is rate determining, whereas the steps prior control the amount of intermediate.
Relate \(k_{r}\) to Activation Energy
Recall from Lecture 4:
For each step of the reaction, we can use the Arrhenius equation to substitute \(k_{r}=Ae^{(-\frac{E_{a}}{RT})}\), where \(A\) is the frequency factor and \(E_{a}\) is the activation energy
So we can express the overall rate constant of the reaction above as:
\[k_{r}=\frac{A_{a}e^{-\frac{Ea,a}{RT}}A_{b}e^{-\frac{Ea,b}{RT}}}{A_{a}'e^{-\frac{Ea,a'}{RT}}}=\frac{A_{a}A_{b}}{A_{a}'}e^{-\frac{(Ea,a+Ea,b-Ea,a')}{RT}}\]
Although each rate constant may increase with temperature, this may not be true of the overall rate constant \(k_{r}\).
The effective activation energy is: \(E_{a}=E_{a}+E_{b}-E_{a}'\) and this may be positive or negative depending on \(E_{a}\) of individual steps.
The two-step mechanism below has been proposed for a reaction between nitrogen monoxide and molecular chlorine:

Use this mechanism to derive the equation and predicted rate law for the overall reaction.
The rate law should be expressed in terms of \([NO]\), \([Cl_{2}]\), and the rate constants of the elementary reactions shown above.\(^{[3]}\)
Solution
Add example text here.
Step 1: The overall reaction is the sum of the 2 elementary reactions:
\[2NO_{(g)}+Cl_{2(g)}→2NOCl_{(g)}\]
Step 2: Write each reaction separately and express the rate law for each reaction (including the reverse reaction in the equilibrium):
\begin{align*}
NO_{(g)}+Cl_{2(g)}→NOCl_{2(g)},\;\;\; & r_{1}=k_{a}[NO][Cl_{2}]\\
NOCl_{2(g)}→NO_{(g)}+Cl_{2(g)},\;\;\; & r_{-1}=k_{a}'[NOCl_{2}]\\
NOCl_{2}+NO→2NOCl,\;\;\; & r_{2}=k_{b}[NOCl_{2}][NO]
\end{align*}
Step 3: Use the equilibrium reaction to express the concentration of the intermediate (\([NOCl_{2}]\)):
\[[NOCl_{2}]=\frac{k_{a}}{k_{a}'}[NO][Cl_{2}]\]
Step 4: Express the overall reaction rate using the step 2 elementary reaction, then subsitute in \([NOCl_{2}]\):
\begin{align*}
overall\;rate&=\frac{1}{2}\frac{d[NOCl]}{dt}\\
&=\frac{2k_{b}[NO][NOCl_{2}]}{2}\\
&=k_{b}[NO][NOCl_{2}]\\
&=\frac{k_{b}k_{a}}{k_{a}’}[NO]^2[Cl_{2}]
\end{align*}
References
[1] Chemistry LibreTexts. 2020. Steady State Approximation. [online] Available at: <https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/Reaction_Mechanisms/Steady-State_Approximation> [Accessed 04 May, 2020].
[2] Introduction to Chemistry. 1st Canadian Edition. 2014. Reaction Mechanisms. [online] Available at: <https://opentextbc.ca/introductorychemistry/chapter/reaction-mechanisms-2/> [Accessed 04 May, 2020].
[3] OpenStax Chemistry 2e. 2019. 12.6 Reaction Mechanisms. [online] Available at: <https://openstax.org/books/chemistry-2e/pages/12-6-reaction-mechanisms> [Accessed 05 May, 2020].

