4.4: Acid Rain and Ozone (I)
- Page ID
- 47174
Acid rain is a serious environmental problem around the world, particularly affecting Asia, Europe, and large parts of the U.S. and Canada. The acidic pollutants such as SO2 and NOx are emitted into the environment by combustion of fossil fuels.
Most of the sulfur in any fuel combines with oxygen and forms SO2 in the combustion chamber. This SO2 when emitted into the atmosphere slowly oxidizes to SO3. SO3 is readily soluble in water in the clouds and forms H2SO4 (sulfuric acid).
\[ \ce{ S + O2 -> SO2 + \dfrac{1}{2}O2 \, (in \, the \, atmosphere) } \nonumber\]
\[ \ce{ SO2 + \dfrac{1}{2} O2 \, (in \, the \, atmosphere) -> SO3 + H2O } \nonumber\]
\[ \ce{ SO3 + H2O -> H2SO4 \, (sulfuric \, acid) } \nonumber\]
Most of the NOx that is emitted is in the form of NO. This NO is oxidized in the atmosphere to NO2. NO2 is soluble in water and forms HNO3 (nitric acid).
\[ \ce{ NO + \dfrac{1}{2} O2 \, (in \, the \, atmosphere) -> NO2 + H2O } \nonumber\]
\[ \ce{ NO2 + H2O -> HNO3 \, (nitric \, acid) } \nonumber\]
Acid Deposition
Sunlight increases the rate of most of the SO2 and NO reactions. The result is a mild solution of sulfuric acid and nitric acid. "Acid rain" is a broad term used to describe several ways that acids fall out of the atmosphere. A more precise term is acid deposition, which has two parts: wet and dry.
- Wet deposition - refers to acidic rain, fog, and snow. As this acidic water flows over and through the ground, it affects a variety of plants and animals. The strength of the effects depend on many factors, including:
- the acidity of the water;
- the chemistry and buffering capacity of the soils involved;
- the types of fish, trees, and other living things that rely on the water.
- Dry deposition - refers to acidic gases and particles. About half of the acidity in the atmosphere falls back to earth through dry deposition.
- Acidic particles and gases are blown by the wind onto buildings, cars, homes, and trees.
- Dry deposited gases and particles can also be washed from trees and other surfaces by rainstorms. When that happens, the runoff water adds those acids to the acid rain, making the combination more acidic than the falling rain alone
Process of Acid Deposition
Prevailing winds blow the compounds that cause both wet and dry acid deposition across state and national borders, and sometimes over hundreds of miles. The video goes into more detail about the process of acid deposition.
pH Scale
Acid rain is measured using a pH scale.
pH is a measure of hydrogen ion concentration, which is measured as a negative logarithm. In other words, acids produce hydrogen ions and alkalis produce hydroxyl ions, so pH is the power of a solution to yield hydrogen ions [H+].
The pH scale ranges from 0 to 14 and indicates how acidic or basic a substance is.
- A pH of 7 is neutral.
- A pH less than 7 is acidic.
- A pH greater than 7 is basic.
The lower a substance's pH, the more acidic it is. Each whole pH value below 7 (the neutral point) is ten times more acidic than the next higher value.
- For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6.
The higher a substance’s pH, the more basic or alkaline it is.
- Each whole pH value above 7 is ten times more alkaline (another way to say basic) than the next lower whole value.
- For example, a pH of 10 is ten times more alkaline than a pH of 9.
Figure 4.4.1 gives a visual of the pH scale.
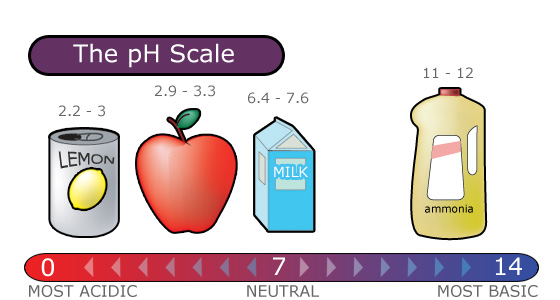
Figure 4.4.1. pH scale
Effects of Acid Rain
Overview
Pure water has a pH of 7.0. Normal rain is slightly acidic because carbon dioxide dissolves into it, so it has a pH of about 5.5. As of the year 2000, the most acidic rain falling in the US has a pH of about 4.3.
Below is a video demonstration that replicates the effect of acid rain on plant life. In the video, beans are placed in: a) water, b) slightly acidic water and c) acidic water, and their growth is observed over a period of three days.
Effects of Acid Rain on Forests and Aquatic Life
Forests
Acid rain does not usually kill trees directly. Instead, it is more likely to weaken trees by:
- damaging their leaves;
- limiting the nutrients available to them;
- exposing them to toxic substances slowly released from the soil.
Quite often, injury or death of trees is a result of these effects of acid rain in combination with one or more additional threats.
Aquatic Life
Acid rain causes acidification of lakes and streams and contributes to damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. Several regions in the U.S. were identified as containing many of the surface waters sensitive to acidification, as shown in Figure 4.4.2. They include the:
- Adirondacks and Catskill Mountains in New York State;
- Mid-Appalachian Highlands along the east coast;
- Upper Midwest;
- Mountainous areas of the Western United States.
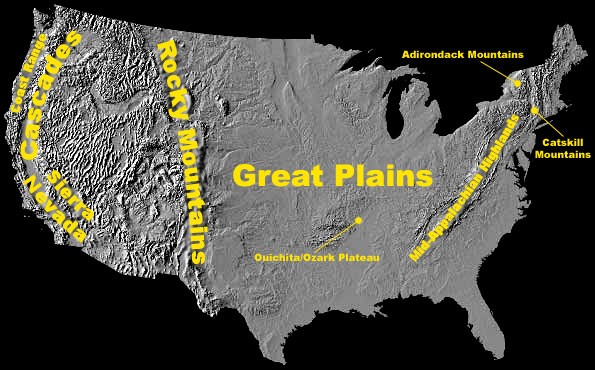
Figure 4.4.2. Map of the U.S. showing high elevation areas
Some types of plants and animals can handle acidic waters. Others, however, are acid-sensitive and will be lost as the pH declines. Figure 4.4.3 shows the pH levels that fish, shellfish, and insects can tolerate.
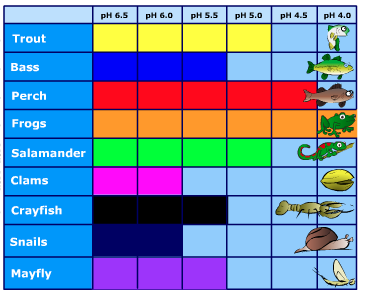
Figure 4.4.3. pH tolerances of various organisms
Effects of Acid Rain on Materials, Visibility, and Human Health
Materials
Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze) and the deterioration of paint and stone (such as marble and limestone). These effects seriously reduce the value to society of buildings, bridges, cultural objects (such as statues, monuments, and tombstones), and cars. Figure 4.4.4 shows an example of a statue affected by acid rain.

Figure 4.4.4. Acid rain erosion to a statue
Visibility
Sulfates and nitrates that form in the atmosphere from sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions contribute to visibility impairment, meaning we can't see as far or as clearly through the air.
Eastern U.S.
Sulfate particles account for 50 to 70 percent of the visibility reduction in the eastern part of the United States, affecting our enjoyment of national parks, such as the Shenandoah and the Great Smoky Mountains.
Through the Acid Rain Program, SO2 reductions will be completed to improve visual range at national parks located in the eastern United States, such as the Great Smoky Mountains, shown in Figure 4.4.5. Based on a study of the value national park visitors place on visibility, these reductions are expected to be worth over a billion dollars annually by the year 2010.

Figure 4.4.5. Great Smoky Mountains
Western U.S.
In the western part of the United States, nitrates and carbon also play roles, but sulfates have been implicated as an important source of visibility impairment in many of the Colorado River Plateau national parks, including the Grand Canyon (shown in Figure 4.4.6), Canyonlands, and Bryce Canyon.

Figure 4.4.6. Grand Canyon
Human Health
Acid rain looks, feels, and tastes just like clean rain. The harm to people from acid rain is not direct. Walking in acid rain, or even swimming in an acid lake, is no more dangerous than walking or swimming in clean water. However, the pollutants that cause acid rain also damage human health.
- Effects of Sulfur Dioxide (SO2): These gases interact in the atmosphere to form fine sulfate and nitrate particles that can be transported long distances by winds and inhaled deep into people's lungs. Fine particles can also penetrate indoors. Many scientific studies have identified a relationship between elevated levels of fine particles and increased illness and premature death from heart and lung disorders, such as asthma and bronchitis.
- Effects of Nitrogen Oxide (NOx): Decrease in nitrogen oxide emissions are also expected to have a beneficial impact on human health by reducing the nitrogen oxides available to react with volatile organic compounds and form ozone. Ozone impacts on human health include a number of morbidity and mortality risks associated with lung inflammation, including asthma and emphysema.
Protecting the Environment from Acid Rain
You can do the following to protect the environment from acid rain:
- Turn off lights, computers, and other appliances when you're not using them.
- Use energy efficient appliances: lighting, air conditioners, heaters, refrigerators, washing machines, etc.
- Only use electric appliances when you need them.
- Keep your thermostat at 68°F in the winter and 72°F in the summer. You can turn it even lower in the winter and higher in the summer when you are away from home.
- Insulate your home as best you can.
- Carpool, use public transportation, or better yet, walk or bicycle whenever possible.
- Buy vehicles with low NOx emissions, and maintain all vehicles well.


