4.5: Acid Rain and Ozone (II)
( \newcommand{\kernel}{\mathrm{null}\,}\)
Introduction to Ozone
Ozone (O3) is a triatomic oxygen molecule gas that occurs both in the Earth’s upper atmosphere and at ground level. Ozone can be good or bad, depending on where it is found: It is a bluish gas that is harmful to breathe. Therefore, it is bad at the ground level. Figure 4.5.1 has more information on ozone.
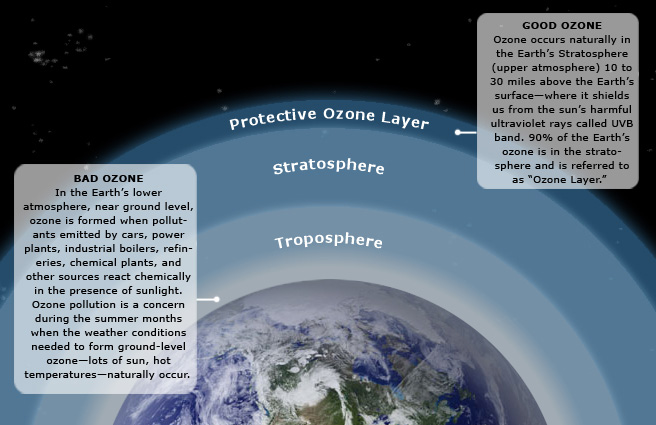
Figure 4.5.1. Ozone in the Earth's atmosphere
The Ozone Cycle
The video below shows the process of ozone depletion. Ozone depletion is caused by chlorofluorocarbons (CFCs) and other ozone-depleting substances.
Production and Destruction of Ozone
Ozone is constantly produced and destroyed in a natural cycle, as shown in the figure below. However, the overall amount of ozone is essentially stable. This balance can be thought of as a stream's depth at a particular location. Although individual water molecules are moving past the observer, the total depth remains constant. Similarly, while ozone production and destruction are balanced, ozone levels remain stable. This was the situation until the past several decades. The following video talks more about ozone destruction.
Large increases in stratospheric chlorine and bromine, however, have upset the balance of the Ozone. In effect, they have added a siphon downstream, removing ozone faster than natural ozone creation reactions can keep up. Therefore, ozone levels fall.
Since ozone filters out harmful UVB radiation, less ozone means higher UVB levels at the surface. The more the ozone is depleted, the larger will be the increase in incoming UVB radiation. UVB has been linked to:
- skin cancer;
- cataracts;
- damage to materials like plastics;
- harm to certain crops and marine organisms.
Although some UVB reaches the surface even without ozone depletion, its harmful effects will increase as a result of this problem.
Additional Information
Ozone-Depleting Substance(s) (ODS) are:
- CFCs;
- HCFCs (used in the energy related to refrigeration and air conditioning in homes, commercial buildings, and cars, and manufacture of foam products);
- Halons (used in fire extinguishers);
- Methyl bromide, carbon tetrachloride;
- Methyl chloroform (used as solvents in chemical industries).
The Ozone Hole
Recent studies by NASA and others have indicated that about 40 percent of the ozone in the Antarctica has been destroyed and that about 7 percent of ozone is destroyed from the Arctic Circle. The destruction of ozone is also called “Ozone Hole."
Ozone hole does not mean that there is no ozone in the region. The ozone hole is defined as the area having less than 220 dobson units (DU) of ozone (concentration) in the overhead column (i.e., between the ground and space).
Figure 4.5.2 and 4.5.3 show the reduction in ozone concentration over Antarctica. This hole in the Antarctica is unfortunately allowing more Australians to be exposed to UV radiation. However, if this kind of ozone destruction ever takes place in the Arctic zone, more humans (in the Northern hemisphere) would be exposed to higher levels of UVB radiation.
Figure 4.5.2. Ozone concentrations in the Antarctic
Credit: Ozone Concentrations in the Antarctic by NASA Ozone Watch, available for public use.
Figure 4.5.3. Map of Antarctica showing total ozone (DU) / ozone total (UD) as of Sept. 12, 2011
Credit: Antarctica Ozone by NASA Ozone Watch, available for public use.
Fun Fact
A Dobson Unit is the measure of the amount or thickness of ozone in the atmosphere. It is based on a measurement taken directly above a specific point on the Earth's surface. One Dobson unit refers to a layer of ozone that would be 0.001 cm thick under conditions of standard temperature (0 degree C) and pressure (the average pressure at the surface of the Earth). The Dobson unit was named after G.M.B. Dobson, who was a researcher at Oxford University in the 1920s. He built the first instrument (now called the Dobson meter) to measure total ozone from the ground.
The size of the Southern Hemisphere ozone hole as a function of the year is shown in the figure below. Figure 4.5.4 compares the size of the hole over a twenty year period, from 1980 to 2010. It can be seen that the size increased each year. Each year in the spring, the ozone hole is at its largest.
Figure 4.5.4. Southern hemisphere ozone hole area
Effects of Ozone Depletion on Skin
Effects of ozone depletion can result in 1) increased cases of skin cancer, 2) skin damage, 3) cataracts and other eye damage, and 4) immune suppression.
Skin Cancer
The incidence of skin cancer in the United States has reached epidemic proportions. One in five Americans will develop skin cancer in their lifetime, and one American dies every hour from this devastating disease.
Medical research is helping us understand the causes and effects of skin cancer. Many health and education groups are working to reduce the incidence of this disease, of which 1.3 million cases have been predicted for 2000 alone, according to The American Cancer Society. Figure 4.3.11 shows the sources of ozone depleting substances.
<insert pie chart>
Figure 4.5.5. Various sources that produce ODS
Melanoma
Melanoma, the most serious form of skin cancer, is also one of the fastest growing types of cancer in the United States. Many dermatologists believe there may be a link between childhood sunburns and melanoma later in life. Melanoma cases in this country have more than doubled in the past 2 decades, and the rise is expected to continue.
Non-melanoma Skin Cancers
Nonmelanoma skin cancers are less deadly than melanomas. Nevertheless, left untreated, they can spread, causing disfigurement and more serious health problems. More than 1.2 million Americans will develop nonmelanoma skin cancer in 2000 while more than 1,900 will die from the disease. There are two primary types of nonmelanoma skin cancers.
- Basal Cell Carcinomas are the most common type of skin cancer tumors. They usually appear as small, fleshy bumps or nodules on the head and neck, but can occur on other skin areas. Basal cell carcinoma grows slowly, and rarely spreads to other parts of the body. It can, however, penetrate to the bone and cause considerable damage.
- Squamous Cell Carcinomas are tumors that may appear as nodules or as red, scaly patches. This cancer can develop into large masses, and unlike basal cell carcinoma, it can spread to other parts of the body.
These two cancers have a cure rate as high as 95 percent if detected and treated early. The key is to watch for signs and seek medical treatment.
Other Skin Damage
Other UV-related skin disorders include actinic keratoses and premature aging of the skin.
- Actinic keratoses are skin growths that occur on body areas exposed to the sun. The face, hands, forearms, and the "V" of the neck are especially susceptible to this type of lesion. Although premalignant, actinic keratoses are a risk factor for squamous cell carcinoma. Look for raised, reddish, rough-textured growths and seek prompt medical attention if you discover them.
- Chronic exposure to the sun also causes premature aging, which over time can make the skin become thick, wrinkled, and leathery. Since it occurs gradually, often manifesting itself many years after the majority of a person's sun exposure, premature aging is often regarded as an unavoidable, normal part of growing older. With proper protection from UV radiation, however, most premature aging of the skin can be avoided.
Note
Protect yourself against sunburn. Minimize sun exposure during midday hours (10 am to 4 pm). Wear sunglasses, a hat with a wide brim, and protective clothing with a tight weave. Use a broad spectrum sunscreen with a sun protection factor (SPF) of at least 15. To be safer, 30 is better.
Effects of Ozone Depletion on Eyes and Immune System
Cataracts and Other Eye Damage
Cataracts are a form of eye damage in which a loss of transparency in the lens of the eye clouds vision. If left untreated, cataracts can lead to blindness. Research has shown that UV radiation increases the likelihood of certain cataracts. Although curable with modern eye surgery, cataracts diminish the eyesight of millions of Americans and cost billions of dollars in medical care each year. The following video provides more information on cataracts.
Other kinds of eye damage include pterygium (i.e., tissue growth that can block vision), skin cancer around the eyes, and degeneration of the macula (i.e., the part of the retina where visual perception is most acute). All of these problems can be lessened with proper eye protection from UV radiation.
Immune Suppression
Scientists have found that overexposure to UV radiation may suppress proper functioning of the body's immune system and the skin's natural defenses. All people, regardless of skin color, might be vulnerable to effects including impaired response to immunizations, increased sensitivity to sunlight, and reactions to certain medications.
Protecting the Environment - Ozone Depletion
Your "power" in protecting the environment from ozone depletion includes:
- Making sure that technicians working on your car air conditioner, home air conditioner, or refrigerator are certified by an EPA-approved program to recover the refrigerant (this is required by law).
- Having your car and home air conditioner units and refrigerator checked for leaks. When possible, repair leaky air conditioning units before refilling them.
- Contacting local authorities to properly dispose of refrigeration or air conditioning equipment.
International Action in Protecting the Environment from Ozone Depletion
In 1987, the Montreal Protocol, an international environmental agreement, established requirements that began the worldwide phase out of ozone-depleting CFCs (chlorofluorocarbons). These requirements were later modified, leading to the phase out in 1996 of CFC production in all developed nations.
Ground Level Ozone and Photochemical Smog
Ozone is a secondary pollutant that forms from the primary pollutants such as Volatile Organic Compounds (Hydrocarbons) and nitrogen oxides (NOx) in the presence of sunlight, as shown in Figure 4.5.6. Its formation is mainly from the automobile emissions.

Figure 4.5.6. Ozone formation
Below is a demonstration on how ozone forms at the ground level (note ground level ozone is also known as “bad” ozone).
As previously mentioned, the formation of ozone is mainly from automobile emission. A typical profile of pollutants in the air of major cities is well repeatable and is shown in the figure below. Note how the formation changes over the course of a day, as shown in Figure 4.5.7:
- Early in the morning - During peak traffic hours, NO and Hydrocarbons are emitted along with CO.
- Mid-morning - NO is slowly oxidized to NO2.
- Mid-afternoon - In the presence of sunlight, NOx react with VOCs to form ozone.
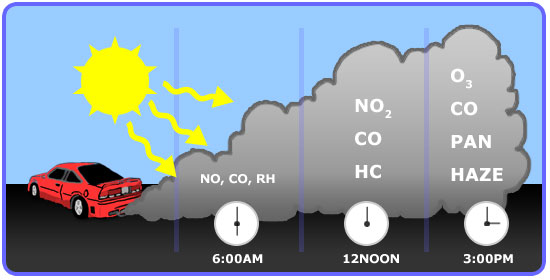
Figure 4.5.7. Typical pollutant profile and ozone formation during the day
Ozone, by itself, is damaging to health and also to the environment. Ozone triggers a variety of health problems even at very low levels and may cause permanent lung damage after long-term exposure. Ozone also leads to the formation of smog or haze, causing additional problems such as a decrease in visibility as well as damage to plants and ecosystems.






