Chapter 1: Introduction, Bonding, and Polymer Chains
- Page ID
- 114948
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Soft Matter/Polymers:
Although we will typically focus on polymers in this course, a majority of the concepts that we will discuss will apply to soft materials/soft matter. The field of soft matter/materials concerns the class of materials which can include polymers, nanoparticles, organic-inorganic hybrid systems, colloids, and liquid crystals. The study of soft matter is particularly relevant as most biological systems are composed primarily if not entirely of soft matter. Thus some of the fundamental principles/physical insights that we will gain into polymers may be applicable more generally to biological systems as well.
Polymers and soft matter have become extremely prevalent everyday life after WWI even though naturally occurring polymers (wood, rubber, silk, cellulose) have existed for centuries. Quick note, the ancient Mayan civilizations not only utilized natural rubbers as materials for sport equipment but also were the first to cross-link rubber as well, long before Charles Goodyear in 1839. Soft materials and polymers are becoming more and more important and are rapidly replacing metallic and other materials in a myriad of applications. We have transitioned from the Stone age to Bronze, Iron, Steel, Silicon (1950), and are currently in the carbon/polymer/plastic age.

Polymers will typically have much lower Young’s modulus, strength, temperature use range, and are poor conductors of electricity and heat. However, polymers are cheap and easy to process, have a lower density, and are more resistant to chemicals that most metals. The are also dynamical at room temperature and are constantly fluctuating and re-configuring bonds which gives rise to self-healing behavior. For many mechanical applications there is a concerted effort to move to materials with a high strength to density ratio. This motivation should be clear for applications in aerospace and electric vehicles as replacing dense materials with light weight alternatives will reduce fuel consumption and thus cost. You can see an example of this in the Ashby plot below, named for Michael Ashby who developed a software which plots a scatter plot in a Log-Log scale to compare properties of different.
It is clear from this plot that polymers and certain natural materials or elastomers can achieve the same strength at an order of magnitude lower density.
Now polymers microstrucually, mechanically, and in many other aspects are much more complicated and difficult to study than metals or ceramics. You will see in this class over and over again that the basic principles you learned in Materials Science for how to analyze metals and ceramics will often have to be modified or an additional wrinkle will have to be added to study polymeric materials or soft matter. However, once you understand polymers you will find your understanding of metals and ceramics will be much better as well. Once you study polymers you can do anything!
Basic Structure/Configuration of Polymers/Soft Matter:
So let’s start with the basics and describe the basic structure of a polymer. In 1920 Staudinger first hypothesized that polymers were not composed of many molecules but instead he believed that polymers were composed of very large molecules containing long sequences of simple chemical units linked by covalent bonds. Staudinger even gave them a name, macromolecules. Macromolecules or polymers are giant chainlike molecules composed of long sequences of one more more species of atoms or groups of atoms (monomer) linked together via covalent bonds. These monomers, also called structural units for protein or peptides, are the basic building blocks of polymer chains as seem below

These are often confused with a repeat unit which is understandable because in certain cases a repeat unit and a monomer or structural unit are the same. A repeat unit is a structural unit or combination of structural units that repeat along a chain.
Let’s do a quick problem with distinguishing between a repeat unit and a structural unit in the three images below identify the number of structural units and then number of repeat units

As you can see for polysytrene we have 1 structural unit and 1 repeat unit. This is a typical scenario that you will see for polymers in some polymerization methods. For nylon 6,6 we can actually see two distinct structural units but again only 1 repeat unit. For the random block copolymer we again see 2 structural units but 0 repeat units. Once we get into the polymerization chemistry next lecture you will be able to start distinguishing/recognizing these different structural units. The process by which many monomers are linked together to form a polymer chain is polymerization. Much more on polymerization chemistry in the next lecture. You can see an example of polystyrene below
Many monomer units and polymers in general are organic and thus are composed of many hydrocarbons, i.e. molecules composed of hydrogen and carbon. Carbon can form 4 single bonds with hydrogen and this is referred to as a saturated hydrocarbon. Here are some common saturated hydrocarbon polymers that you may come across.
If there are double or triple bonds then we are dealing with unsaturated hydrocarbons, the implications of this will be important when discussing lipid bilayers, Tg, and other polymer concepts. A polymer chain can be composed of less than 100 monomers is referred to as a oligomers (waxy polymers). However polymers

are typically composed of up to 10-100k monomers. Polytheylene for example is typically composed of 104 monomers. Additionally the monomers may typically be composed of other functional groups or molecules that are a bit more exotic than hydrocarbons and this will imbue some very unique properties to these polymers. Some of the functional groups you may come across can be seen below (no need to memorize them but become a familiar with them to brush up on your chemistry).

Note that R just stands for some arbitrary side group that can be attached, we will discuss this much more in detail next lecture when we discuss polymerization. Additionally here are some common polymers that we will encounter in this class as well as in the real world.

Now we have mentioned the term chain quite a bit when describing the basic structure of a polymer and we will continue to use this description as many of the scenarios that we will discuss involves treating the polymer like a random coiled chain, like spaghetti. However, polymer structure/architecture can vary dramatically and the structure can have implications for the polymer properties. Polymers can be
- Linear i.e. polyethylene (PE), polyvinyl chloride (PVC), polymethyl methacrylate (PMMA)
- Branched
- Crosslinked/Network
- Ladder
- Comb
- Star
- Dendrimer
- Copolymer
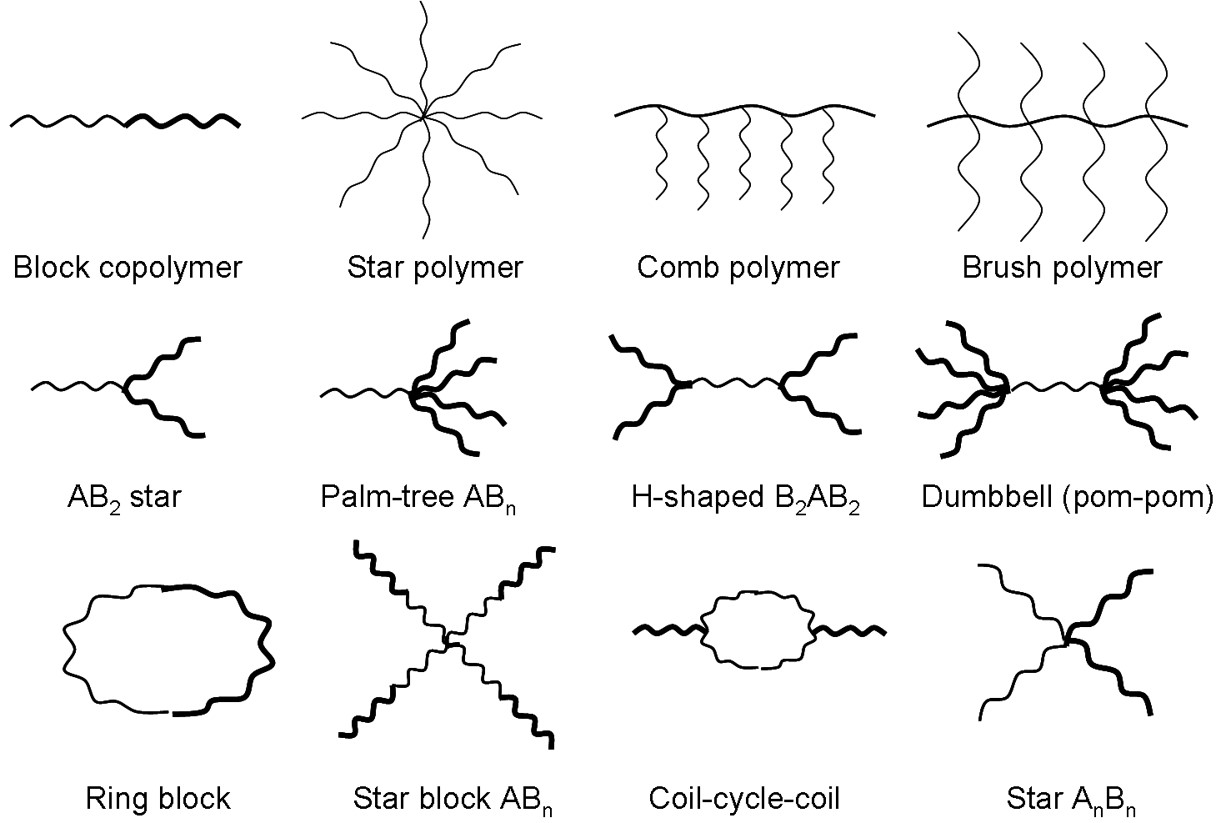
A linear polymer can also be cyclic or closed linear or like a rigid rod. The crosslinked structure will have very different properties (mechanical) depending on type and number of crosslinks. The copolymer structure is one we will return to again later on in the course when we discuss block, random, and graft copolymers.
Polymer Configurations:
We have talked about different polymer structures but polymers will also often adopt different configurations depending on a variety of factors like temperature, environment, solvent, and external stimuli. Some of these configurations are listed and seen below
- Random Coil
- Folded Chain
- Extended Chain
- Fringed Micelle

Basic Types/Classification of Polymers:
In addition to structure we can also classify polymers more broadly as being in three distinct groups
- Thermoplastics
- Thermosets
- Elastomers
A thermoplastic polymer, the polymer we will discuss most often, can be a linear, branched, comb, star, dendrimer, or copolymer which will soften or melt upon heating. On cooling they will harden and this process of softening and hardening is reversible and can be repeated. As the temperature increases the intermolecular bonds are broken, i.e. the secondary bonds not the covalent bonds. Upon cooling the entire polymer will not crystallize due to the unfavorable energetics of ordering of highly coiled polymer chain. However, some regions of the polymer will form semi-crystalline regions where both crystalline and amorphous regions exist. These semi-crystalline polymers are characterized by their melting temperature, Tm. However, many polymers are completely amorphous and cannot from any crystalline regions and these polymers are characterized by their glass transition temperature, Tg, which the temperature which indicated the onset of cooperative segmental mobility. Cooperative segmental mobility refers to the cooperative motion of 10-100’s of polymer repeat units. You can also think about the glass transition as the transition from a glassy state (hard) to a more rubbery state (soft). Much more on glass transitions later. Thermoplastics can be easily fabricated via extrusion/injection and include polymers such as PE, polystyrene (PS), PET, PVC, PMMA.

A thermoset polymer are typically network, crosslinked, or ladder polymers. Due to the permanent (covalent) crosslinks between molecular chains they do not soften upon heating. Due to the high degree of crosslinking (10-50%) the permanent crosslinks resist molecular motion at high temperatures and will eventually degrade at extremely high temperatures due to breaking of crosslinks. This is not a reversible transition. Some examples of thermosets are vulcanized rubbers, epoxies, phenolics, and some polyester resins.
An elastomer is somewhat similar to a thermoset polymer but has some key distinctions. These polymers are also crosslinked but not to the degree of thermoset polymers, much less. Additionally, elastomers can be stretched very easily up to 10× their original dimensions and recover once the stress is relieved due to an entropic restoring force. Some examples of elastomers are rubbers like polybutadiene (BR) or styrene-butadiene rubber (SBR).
Bonding/Molecular Interactions: Intra vs Intermolecular Forces
So thus far we have talked a lot about polymer structure and configuration but we have not talked about what makes soft matter unique. The key to most of the fascinating properties of soft materials lies in intermolecular interactions in soft matter systems. Here we need to take a step back and define the difference between intra and inter molecular interactions. Intra (within) molecular interactions are interactions that occur within the molecule or polymer chain, i.e. the covalent backbone interactions for polymers (also generally covalent, ionic, or metallic). Inter (between) molecular interactions occur between molecules and other types of neighboring particles. These are typically secondary interactions, more on this in a bit. These secondary interactions, intermolecular interactions, are typically much weaker than the intramolecular interactions but these interactions are critical for some of the most unique properties of soft matter. In fact most of the intermolecular interactions in soft matter have energies, or enthalpies, on the order of approximately 1 kT, where k is the Boltzmann constant T is the temperature of the system. The implication here is that soft matter systems are held together by very weak intermolecular interactions and this will lead to large fluctuations in behavior because these bonds can be easily broken by applying an external stimulus, like pulling a polymeric material. This is in stark contrast to hard materials which are held together by covalent bonds which are typically 10-200kT. These weak intermolecular interactions in soft matter also leads to polymers being very adaptive and can undergo large changes in response to fairly weak stimuli. This behavior is critically important in biological systems where rapid protein folding or conformational changes in receptors is critical for biological processes. This is only possible because of these weak interactions. However, don’t get it mistaken that all polymers behave like silly putty. These interactions are weak but there are a very large number of these interactions and from this you can build up materials like Kevlar, which derives much of it’s mechanical properties from the structure of the polymer backbone. Again, this will remain the focus of this class which is to relate macroscopic behavior to microscopic properties, or in other words to relate the molecular structure, bonding, and conformational changes to macroscopic behavior, similar to the Materials Tetrahedron
Intramolecular Forces/Interactions:
When it comes to binding and types of intramolecular interactions it is all about the electrons. Elements that have no valence electrons or that have a completely filled

outer shell are called the inert or noble gases. All other elements which do not have a completely filled outer shell have valence electrons and these electrons are the ones of import when it comes to bonding. Bonding is critically important as most of the structural, physical, and chemical properties are influenced or determined by the interatomic bonding.
For intramolecular interactions we will typically deal with
- Covalent (5eV or about 200 kT)
- Ionic (1-3eV or 80kT)
- Metallic (0.5eV or 20kT)
Let’s talk a little bit about why bonding occurs. Well it all goes back to Gibbs, the energy of the set of bonded atoms is lower than the isolated atoms. There are forces of attraction and repulsion between electrons and protons. And we remember that the force (F) is related to the potential energy (U) by F = −∇U. Remember that ∇ is the gradient mathematical operator and will take the partial derivative of the function with respect to the dimensionality of the problem. Now there are many different equations or potentials that describe the interactions between atoms i.e. Morse, Born-Mayer, Van Der Waals, etc. Let’s take a look at one of these potentials, the Lennard-Jones (LJ) Potential which approximates the interaction between a pair of neutral atoms (it is popular due to the computational simplicity)
\[
V_{LJ} = 4\epsilon \left[\left(\frac{\sigma}{r} \right)^{12} - \left(\frac{\sigma}{r} \right)^{6}\right] = \epsilon \left[\left(\frac{r_{o}}{r} \right)^{12} - 2\left(\frac{r_{m}}{r} \right)^{6}\right]
\nonumber \]
where is the depth of the potential well (energy), σ is the distance at which the inter-particle potential is zero, r is the distance between particles, and rm is the distance where the potential is minimized.
We can distinguish between different types of intramolecular interactions and how to distinguish between covalent, ionic, and metallic interactions. Well, the key to determining which type of bonding occurs is the valence electrons. Specifically if they are gained, lost or shared.
With covalent bonds there are valence electrons that are shared between adjacent atoms, which results in a pairing of electrons into localized orbitals and concentrating the negative charge between the positive nuclei. For ionic interactions we are typically dealing with atoms that have a different affinity for electrons, different electronegativities, a net transfer of charge can occur forming positively and negatively charged ions. These ions can form networks of ionic bonds held together by long-range coulombic interactions.
When the electronegativity is similar or the same between atoms the valence electrons are shared equally and the bond is purely covalent. When the electronegativity is very different the more electronegative atom withdraws nearly all the valence electrons and the bond is purely ionic. Thus electronegativity is how we distinguish between Covalent and Ionic interactions! Now you might ask what happens in the intermediate case of electronegativity differences. Well then you will have bonds that have both covalent and ionic characteristics. We call these bonds polar covalent and one atom will have a partial positive and negative charge, as in HCl. While there is no universally agreed upon cutoff values in this class we will define an electronegativity difference of 0-0.4 to be non-polar covalent, 0.5-1.7 to be polar covalent, and greater than 1.7 to be ionic. In the case of metallic bonds, all atoms share their valence electrons and the nuclei form a positively charged array in a sea of delocalized electrons.
For soft matter the most common intramolecular interaction that we will deal with will be covalent interactions.
Intermolecular Forces/Interactions:
As we previously mentioned intermolecular interactions are critical for many soft matter systems and there are several weak intermolecular interactions of interest, which include:
- Hard sphere potentials - the ability of atoms to effectively occupy space and repel at short length scales, quantum mechanical in nature
- Coulombic/electrostatic interactions - electrostatic attraction/repulsion between charged ions
- Lennard-Jones (LJ) potentials - induced dipole interactions between neutral atoms
- Dipole-dipole interactions - associated with polar molecules and forces
- Hydrogen bonding - net dipole interactions. Typical hydrogen bond donors will be NH groups and possibly OH groups while H-bond acceptors will be electrongative oxygens i.e. -O- or -CO-.
- Hydrophobic effect - a largely entropic interaction related to the structuring of water around hydrophobic interfaces.
- Van der Waals force - includes London dispersion force, Debye force, and Keesom force which describe attraction and repulsion due to fluctating polarization between nearby atoms/elements/particle, quantum mechanical in nature. Van der Waals forces decay as d−6
- Ionic bonding - ionic bonding mediated by metallic ions between side chains You can see some of these interactions illustrated here
If you are interested in the origin of these interactions there is an excellent book by Israelsachvili I can recommend but the key thing to focus on is that all of these interactions have energies on the order of 1kT, so thermal fluctuations can break these bonds potentially.
Isomeric States:
Molecules are typically held together by strong covalent bonds with many conformations available via rotational isomeric states. In other words, conformations are accessible due to the rotation of atoms around these intramolecular bonds. Isomers/Isomeric States are molecules which are compositionally identical but structurally distinct. Conformers/Conformational Isomers are related by

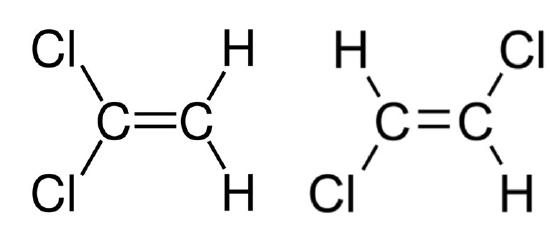
rotations around single bonds. Note that at room temperature thermal rotation around a double or triple bond is essentially zero. There are structural isomers, stereoisomers, geometric/configurational, and sequence isomers. Structural isomers occur when certain bonds prohibit rotoconversion of two conformationally distinct states. Let’s take a look at an example.
Dichlorethene is a structural isomer or a geometric isomer. We can see the cis and trans conformations in the figure. Here the double bond prevents rotational so they are structurally distinct. It is a structural isomer not a conformer.
Stereoisomers have an ordered sequence of linked units but the substituents pendant to the main backbone of covalent bonds have different arrangements or tacticity. Specifically they can be atactic, isotactic, or syndiotactic.
Stereoisomers are found in many different types of polymers and we can see an example of atactic, isotactic, and syndiotactic below:
Isotactic stereoisomers will have all the side-chains on the same side. Syndiotactic will have some type of repeated order either top and bottom, or top-top, bottom-bottom, etc. Atactic is completely random.

A geometric/configurational/cis-trans isomer has the same chemical formula but typically the arrangements of the side groups are on different sides of an unsaturated carbon backbone bond. Specifically there will be cis and trans configurations.
Sequence isomers have many linked units like in a polymer but can have a variable sequence. For example think about block co-polymers which can be designed to be ABABABAB or AAAABBBB. One example of a conformer is dichloroethane. This molecule has a single C-C bond so there are several different rotational states that can be accessed. We can


In the figure we can identify some critical states. The lowest energy states, A, is the trans conformation as the methyl groups are the furthest distance apart from each other. The highest energy state, D, is the cis conformation and the energy is the largest because the methyl groups are the shortest distance from one another. The other two lower energy states, B, are termed gauche ± conformations where there is larger amount of distance between the methyl group than in the cis conformation or the final conformation which is called the eclipsed conformation. The eclipsed conformation, C, where the methyl group is eclipsed by one of the side hydrogens. Molecules like butane can change conformations up to 1010× depending on the temperature.


