6.2: Basic principles
- Page ID
- 7815
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The electrochemical series
Different metals (and their compounds) have different affinities for electrons. When two dissimilar metals (or their compounds) are put in contact through an electrolyte, there is a tendency for electrons to pass from one material to another. The metal with the smaller affinity for electrons loses electrons to the material with the greater affinity, becoming positively charged. The metal with the greater affinity becomes negatively charged. A potential difference between the two electrodes is thus built up until it balances the tendency of the electron transfer between the metals. At this point the potential difference is the equilibrium potential: the potential at which the net flow of electrons is 0.
The electrochemical series represents the quantitative expression of the varying affinity of materials relative to each other. In an aqueous electrolyte the standard electrode potential for an electrode reaction is expressed with respect to a reference electrode. Conventionally this is the H2/H+ cell, with reaction:
\[\mathrm{H}^{+}+\mathrm{e} \leftrightarrow 1 / 2 \mathrm{H}_{2}\]
| Reaction | Eo (V) |
|---|---|
| Li+ + e– → Li |
-3.10
|
| Na+ + e– → Na |
-2.71
|
| Mg2+ + 2e– → Mg |
-2.36
|
| ½H2 + e– → H– |
-2.25
|
| Mn2+ + 2e– → Mn |
-1.18
|
| MnO2 + 2H2O + 4e– → Mn + 4OH– |
-0.98
|
| 2H2O + 2e– → H2 + 2OH– |
-0.83
|
| Cd(OH)2 + 2e– → Cd + 2OH– |
-0.82
|
| Zn2+ + 2e– → Zn |
-0.76
|
| Ni(OH)2 + 2e– → Ni + 2OH– |
-0.72
|
| Fe2+ + 2e– → Fe |
-0.44
|
| Cd2+ + 2e– → Cd |
-0.40
|
| PbSO4 + 2e– → Pb + SO42– |
-0.35
|
| Ni2+ + 2e–→ Ni |
-0.26
|
| MnO2 + 2H2O + 4e– → Mn(OH)2 + 2OH– |
-0.05
|
| 2H+ + 2e– → H2 |
0.00
|
| Cu2+ + e– → Cu+ |
+0.16
|
| Ag2O + H2O + 2e– → 2Ag + 2OH– |
+0.34
|
| Cu2+ + 2e– → Cu |
+0.34
|
| O2 + 2H2O + 4e– → 4OH– |
+0.40
|
| 2NiOOH + 2H2O + 2e– → 2Ni(OH)2 + 2OH– |
+0.48
|
| NiO2 + 2H2O + 2e– → Ni(OH)2 + 2OH– |
+0.49
|
| MnO42– + 2H2O + 2e– → MnO2 + 4OH– |
+0.62
|
| 2AgO + H2O + 2e– → Ag2O + 2OH– |
+0.64
|
| Fe3+ + e– → Fe2+ |
+0.77
|
| Hg2+ + 2e– → Hg |
+0.80
|
| Ag+ + e– → Ag |
+0.80
|
| 2Hg2+ + 2e– → Hg+ |
+0.91
|
| O2 + 4H+ + 4e– → 2H2O |
+1.23
|
| ZnO + H2O + 2e– → Zn + 2OH– |
+1.26
|
| Cl2 + 2e– → 2Cl– |
+1.36
|
| PbO2 + 4H+ + 2e– → Pb2+ + 2H2O |
+1.47
|
| PbO2 + SO42– + 4H+ + 2e– → PbSO4 + 2H2O |
+1.70
|
| F2 + 2e– → 2F– |
+2.87
|
Cells using aqueous electrolytes are limited to under 2 V as water is decomposed at higher voltages. Thus Li batteries available in the 2.7 to 4 V use non-aqueous electrolyte. Typical non-aqueous electrolytes have higher impedance unless used as very thin films, eg lithium polymer battery or at higher temperatures.
For more information on this, click here.
What is a battery?
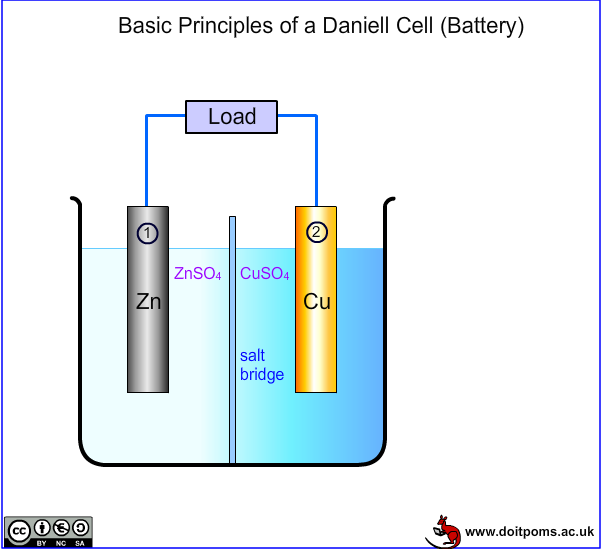
A battery is an electrochemical cell that converts chemical energy into electrical energy. It comprises of two electrodes: an anode (the positive electrode) and a cathode (the negative electrode), with an electrolyte between them. At each electrode a half-cell electrochemical reaction takes place, as illustrated by the animation below.
Discharge
Electrode 1 is an anode: the electrode is oxidised, producing electrons. Electrode 2 is a cathode: the electrode is reduced, consuming electrons. In the fully charged state, there is a surplus of electrons on the anode (thus making it negative) and a deficit on the cathode (thus making it positive). During discharge, electrons therefore flow from the anode to the cathode in the external circuit and a current is produced. Therefore in simple terms batteries work as electron pumps in the external circuit, preferably with only ionic current flowing through the electrolyte. The electrical potential difference between the cathode and the anode, which can drive the electrons in the external circuit, is called electromotive force (emf). Once all the active material at the cathode has been reduced, and/or all the active anodic material is oxidised, the electrode has effectively been used up, and the battery cannot provide any more power. It can then be either disposed of or preferably recycled if it is a primary battery, or recharged if it is a rechargeable (secondary) battery.
If the anode were zinc and the cathode were copper the half reactions would proceed as follows:
At the anode: Zn → Zn2+(aq) + 2e– Eo = 0.76V
At the cathode: Cu2+(aq) + 2e– → Cu Eo = 0.34V
Thus the total potential for this cell is 1.10 V.
During use as a battery, discharge leads to dissolution of Zn at the anode and the deposition of Cu at the cathode. Such a cell is embodied in the Daniell Cell introduced in 1836. As a practical cell this required two electrolytes (typically zinc sulphate and copper sulphate aqueous solutions) to avoid polarisation. The electrolytes are separated from each other by a salt bridge or a porous membrane, which allows the sulphate ions to pass and carry the ionic current, but blocks metallic ions. The Daniell Cell is an effective battery but not practical for portability. More recently, however, the idea of using two separate electrolytes has been resurrected in the form of redox batteries.
Discharge
Electrode 1 is an anode: the electrode is oxidised, producing electrons. Electrode 2 is a cathode: the electrode is reduced, consuming electrons. In the fully charged state, there is a surplus of electrons on the anode (thus making it negative) and a deficit on the cathode (thus making it positive). During discharge, electrons therefore flow from the anode to the cathode in the external circuit and a current is produced. Therefore in simple terms batteries work as electron pumps in the external circuit, preferably with only ionic current flowing through the electrolyte. The electrical potential difference between the cathode and the anode, which can drive the electrons in the external circuit, is called electromotive force (emf). Once all the active material at the cathode has been reduced, and/or all the active anodic material is oxidised, the electrode has effectively been used up, and the battery cannot provide any more power. It can then be either disposed of or preferably recycled if it is a primary battery, or recharged if it is a rechargeable (secondary) battery.
If the anode were zinc and the cathode were copper the half reactions would proceed as follows:
At the anode: Zn → Zn2+(aq) + 2e– Eo = 0.76V
At the cathode: Cu2+(aq) + 2e– → Cu Eo = 0.34V
Thus the total potential for this cell is 1.10 V.
During use as a battery, discharge leads to dissolution of Zn at the anode and the deposition of Cu at the cathode. Such a cell is embodied in the Daniell Cell introduced in 1836. As a practical cell this required two electrolytes (typically zinc sulphate and copper sulphate aqueous solutions) to avoid polarisation. The electrolytes are separated from each other by a salt bridge or a porous membrane, which allows the sulphate ions to pass and carry the ionic current, but blocks metallic ions. The Daniell Cell is an effective battery but not practical for portability. More recently, however, the idea of using two separate electrolytes has been resurrected in the form of redox batteries.
Charge
When the cell potential is depleted the battery can be recharged. When a current is applied to the cell in the opposite direction the anode becomes the cathode, and vice versa. Thus electrode 2 that was oxidised upon discharge is now reduced and the electrode 1 that was reduced is now oxidised so the electrodes are returned to their former state, ready to be discharged again.
This time the anode would be copper and the cathode would be zinc, and the half reactions would proceed as follows:
At the anode: Zn2+(aq) + 2e– → Zn Eo = -0.76V
At the cathode: Cu → Cu2+(aq) + 2e– Eo = -0.34V
The minimum potential required for charging will be 1.10 V, as this is the potential of the cell. In reality much higher potentials will be required to overcome the polarisation.
Example \(\PageIndex{1}\)
How to make a potato battery?
Equipment:
- A potato
- A clean copper coin (If necessary clean it by placing it in a fizzy drink for a few minutes, or clean with steel wool)
- Some aluminium foil
- Crocodile clips
- Wires
- A voltmeter or multimeter
Instructions:
Cut the potato in half and place the flat end on the foil. Push the copper coin into the potato. Attach crocodile clips to the coin and the foil, then wires to the crocodile clips. Now attach these wires to the voltmeter. The volt meter should give a reading, showing that the stored energy is available and a current can flow, and that you have produced a battery!
Why this happens?
The potato contains a mild phosphoric acid (H3PO4), which acts as the electrolyte.
At the copper coin there are two reactions that could take place. If there is sufficient oxygen present, oxygen ions will be reduced to oxygen gas in the reaction
O2 + 4H+ + 4e– → 2O2– + 2H2O 1.23 V
If there is not sufficient oxygen, hydrogen ions from the acid are reduced to hydrogen gas in the reaction
H+ + e– → ½H2 0 V
At the foil aluminium metal is reduced to aluminium ions in the reaction
Al → Al3+ + 3e– 1.66 V
The total theoretical voltage across the potato cell is therefore 2.89 V in sufficient oxygen (Aluminium/air battery), or 1.66 V in insufficient oxygen (Aluminium/Hydrogen battery).
This also works with lemons, tomatoes, apples and other fruits!


