6.11: Lithium batteries
- Page ID
- 21175
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the main attractions of lithium as an anode material is its position as the most electronegative metal in the electrochemical series combined with its low density, thus offering the largest amount of electrical energy per unit weight among all solid elements. In many applications the weight of the battery is a significant percentage of the total weight, and there is great competition to make lighter batteries.
Li cannot be used with the traditional aqueous electrolytes due to the very vigorous corrosive reaction between Li and water with flammable hydrogen as the product. It took many years to develop a suitable electrolyte based on organic solvents with sufficient stability and ionic conductivity. Ionic conductivity is induced by dissolving a suitable Li salt in an organic solvent used in the form of a gel or immobilised within a polymeric separator. In the 1980s progress was made in the use of Li as an anode material with MnO2, liquid SO2 or thionyl chlorides as the cathode, and hexaflurophosphate dissolved in propylene carbonate as a typical organic electrolyte. Li cells are generally properly sealed against contact with air and moisture Whilst the primary lithium battery has been well established for nearly two decades, there have been many problems experienced whilst developing rechargeable lithium batteries, mainly due to the extreme reactivity of lithium, and they have only become widely available since mid 1990s.
Rechargeable batteries
Li-ion batteries are now used in very high volumes in a number of relatively new applications, such as in mobile phones, laptops, cameras and many other consumer products. The typical Li-ion cells use carbon as the anode and LiCoO2 or LiMn2O4 as the cathode. The first commercial Li-ion cell introduced by Sony in the 90's used a polymeric gel electrolyte, swollen by a high proportion of organic solvent for the Li salt. The translucent gel achieved a respectable ionic conductivity of 3×10-3 S cm-1 at 300 K.
Chemistry and construction
In order to overcome the problems associated with the high reactivity of lithium, the anode material is not purely the metal, it is a non-metallic compound, e.g. carbon, which can store and exchange lithium ions. A lithium ion-accepting material, for example CoO2, is then used as the cathode material, and lithium ions are exchanged back and forth between the two during discharging and charging. These are called intercalation electrodes.
This type of battery is known as a “rocking chair battery” as the ions simply “rock” back and forth between the two electrodes.
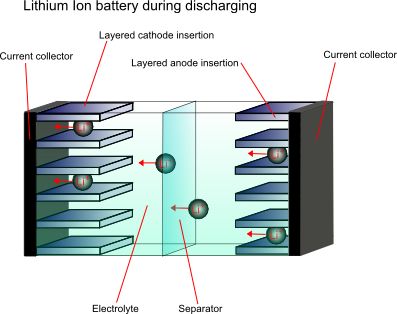
Cathode materials
The most common compounds used for cathode materials are LiCoO2, LiNiO2 and LiMn2O4. Of these, LiCoO2 has the best performance but is very high in cost, is toxic and has a limited lithium content range over which it is stable. LiNiO2 is more stable, however the nickel ions can disorder. LiMn2O4 is generally the best value for money, and is also better for the environment.
Anode material
The anode material is carbon based, usually with composition Li0.5C6. This lithium content is lower than would be ideal, however higher capacity carbons pose safety issues.
Electrolyte
Since lithium reacts violently with water, and the cell voltage is so high that water would decompose, a non-aqueous electrolyte must be used. A typical electrolyte is LiPF6 dissolved in an ethylene carbonate and dimethyl carbonate mixture.
After initial charging the following reactions take place upon discharge:
At the cathode: xLi+ + Mn2O4 + xe- → LixMn2O4
At the anode: LixC6→ xLi+ + 6C + xe-
Overall: LixC6 + Mn2O4 → LixMn2O4 + 6C
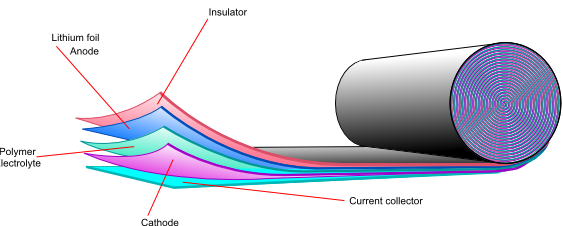
Lithium polymer batteries
Another way of overcoming the high reactivity of lithium is to use a solid polymer electrolyte. Using lithium metal gives a higher energy density, higher cell potential and very low self discharge, so if the safety issues can be overcome, it would be the preferred anode material. Another problem to overcome is the high resistivity of the polymer electrolyte. One possible solution is to use the electrolyte as a very thin film to decrease the total resistance.
Cell capacity and specific energy density
It is important to specify the exact steps taken when calculating the theoretical cell capacity and the maximum specific energy density of a given lithium cell. For full lithium utilization, the cell capacity is 3860 mAh/g of lithium, simply calculated by Faraday’s laws. Thus, the actual rated capacity of the cell in mAh is determined by the weight of lithium in the cell. The actual specific capacity, on the other hand, is usually calculated as the actual rated capacity divided by the weight of lithium in the cell (and quoted as mAh/g of Lithium) or, less frequently, as the ratio of the rated capacity and the weight of the cell (and quoted as mAh/g of the cell). In a general case, the cell weight can be calculated as follows:
Lithium cell capacity and specific energy density
\[\mathrm{W}_{\mathrm{cell}}=w_{\mathrm{Li}} f_{\mathrm{A}}+w_{\mathrm{Li}} f_{\mathrm{C}}+w_{\mathrm{aux}}\]
where
wLi is the weight (wt.) of lithium in the cell;
fA is the multiplier for the anode wt.;
fC is the multiplier for the cathode wt.;
waux is the auxiliary wt. of the cell (which includes the wt. of the electrolyte, separator, connectors etc. and typically comprises of 20% of the wt. of the active part of the cell).
Thus, as a first approximation:
\[W_{\text {cell }} \approx 1.2 w_{\mathrm{Li}}\left(f_{\mathrm{A}}+f_{\mathrm{C}}\right)\]
And the specific capacity of the cell (as mAh/g of the cell wt.) is given by:
\[w_{\mathrm{Li}} 3860 / 1.2 w_{\mathrm{Li}}\left(f_{\mathrm{A}}+f_{\mathrm{C}}\right)=3860 / 1.2\left(f_{\mathrm{A}}+f_{\mathrm{C}}\right)\]
The specific energy density (which is energy density, E divided by the weight of the cell) is the product of the specific capacity and the operating voltage in one full discharge cycle. The values are often quoted for an average voltage and less frequently for the maximum voltage in the discharge sequence. For a constant current drain I, the specific energy density can be written as:
\[\left[I w_{\mathrm{Li}} \int V \mathrm{d} t\right] / w_{\mathrm{cell}}\]
The integration is carried out over the full specified discharge time. The specified discharge time is related to a maximum and a minimum voltage threshold value to allow for recharging the battery. Outside the threshold values, the battery may attain an irreversible state. The specific energy density (Wh/kg of the cell) is related to the specific capacity as follows:
Specific energy density = specific capacity × average operating voltage
\[=w_{\mathrm{Li}} 3860 / 1.2 w_{\mathrm{Li}}\left(f_{\mathrm{A}}+f_{\mathrm{C}}\right) V_{\mathrm{ave}}\]
\[=3860 / 1.2\left(f_{\mathrm{A}}+f_{\mathrm{C}}\right) V_{\text {ave }}\]
Li-ion battery
In order to maximize the specific energy density, it is desirable to minimize the weight of the cell, while maximizing the ratio of weight of lithium to the weight of the cell. For the Li-ion cell, for example, the theoretical stoichiometric value of the anodic multiplier (fA) is 10.3, while for the cathode (fC) is 25. Thus the maximum theoretical specific energy density for a max 4.2 V is calculated to be between 380 to 460 Wh/kg, depending upon whether the weight of the auxiliary components are taken into account.
The stoichiometric value for the carbon anode arises from the fact that lithium is intercalated into the carbon structural layers at the max possible molar ratio of 1 Li atom to 6C atoms giving rise to the limiting formula of LiC6. In practice, Li availability in the anode is only 50% of the theoretical maximum corresponding to the formula Li0.5-xC6 where x can vary from 0 (fully charged state) to 0.5 (fully discharged state). For the cathode, Li is intercalated into a perovskite structure of LiCoO2. Although it appears that, theoretically, the maximum ratio of Li to CoO2 is one, in practice the formula corresponds to Li0.5+xCoO2, where x=0 corresponds to the fully charged state and x=0.5 corresponds to the fully charged state. Thus the available capacities for both the anode and the cathode are more than halved, with the multipliers typically being, fA>21 and fC>50. The excess value arises from the weight of the binder and other additives. The operating voltage during discharge decreases from a max value of 4.2 to a cut-off value of 2.8 V, giving an average value of 3.35 V over the discharge cycle. The practical specific energy density is therefore in the region of 160 Wh/kg for a Li-ion cell.
The only practical method for increasing the specific energy density of a Li-ion cell is to decrease the weight of the auxiliary components of the cell. It is widely believed that with a considerable amount of research and development the maximum specific energy density that can be achieved for a Li-ion cell within the next five years will reach 220 Wh/kg of the cell.
The cycle life of Li-ion batteries are between 500 to 1000 cycles.


