6.10.1: Lead/acid batteries
- Page ID
- 21174
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The lead acid battery is the most used battery in the world. The most common is the SLI battery used for motor vehicles for engine Starting, vehicle Lighting and engine Ignition, however it has many other applications (such as communications devices, emergency lighting systems and power tools) due to its cheapness and good performance.
It was first developed in 1860 by Raymond Gaston Planté. Strips of lead foil with coarse cloth in between were rolled into a spiral and immersed in a 10% solution of sulphuric acid. The cell was further developed by initially coating the lead with oxides, then by forming plates of lead oxide by coating an oxide paste onto grids. The electrodes were also changed to a tubular design.
Characteristics in brief (for an SLI battery)
Voltage: 2 V
Discharge characteristics: Generally quite curved, particularly at higher discharge rate. Best performance with intermittent discharge.
Service Life: Several years
Chemistry
The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte.
The following half-cell reactions take place inside the cell during discharge:
At the anode: Pb + HSO4– → PbSO4 + H+ + 2e–
At the cathode: PbO2 + 3H+ + HSO4– + 2e– → PbSO4 + 2H2O
Overall: Pb + PbO2 +2H2SO4 → 2PbSO4 + 2H2O
During the charging process, the reactions at each electrode are reversed; the anode becomes the cathode and the cathode becomes the anode.
Gassing
During charging, given the high voltage, water is dissociated at the two electrodes, and gaseous hydrogen and oxygen products are readily formed leading to the loss of the electrolyte and a potentially explosive situation. Sealed batteries are made safer by allowing the gases to recombine within the cell.
Sulphation
Under certain circumstances the lead sulphate products at both the electrodes achieve an irreversible state, making the recharging process very difficult.
Construction
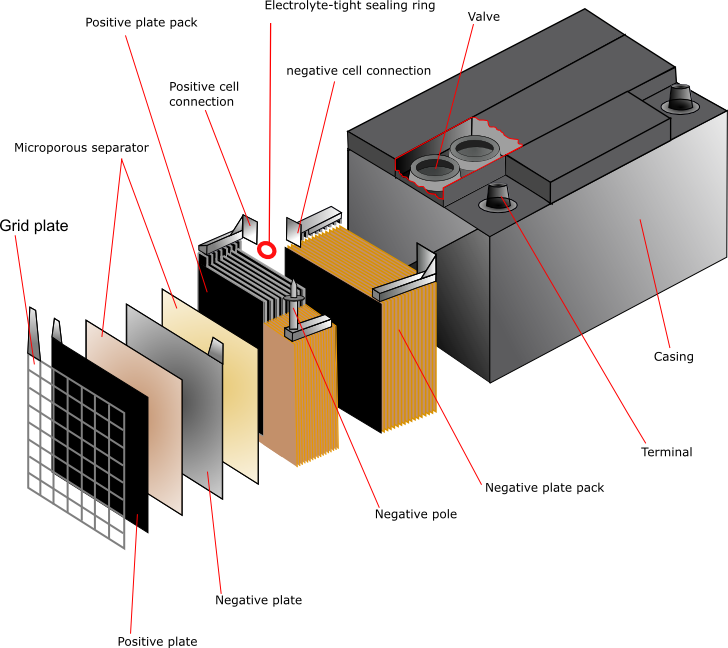
Lead
Pure lead is too soft to use as a grid material so in general the lead is hardened by the addition of 4 – 6% antimony. However, during the operation of the battery the antinomy dissolves and migrates to the anode where it alters the cell voltage. This means that the water consumption in the cell increases and frequent maintenance is necessary. There are two possible solutions to this problem:
(1) Using below 4% the battery water consumption is reduced, however it is then necessary to add small amounts of other elements such as sulphur, copper, arsenic and selenium. These act as grain refiners, decreasing the grain size of the lead and thereby increasing its hardness and strength.
(2) Alkaline earth metals such as calcium can be used to stiffen the lead. This is often used for telephone applications, and for no maintenance automotive batteries, since a more stable battery is required. A typical alloy would be 0.03 – 0.10% calcium and 0.5 – 1.0% tin (to enhance mechanical and corrosion properties).
The function of the grid is to hold the active material and to conduct electricity between the active material and the battery terminals. The design is a simple grid framework with a “tab” or “lug” for connection to the terminal post.
“Book mold” casting is the most common method of production for the grid. Permanent steel molds are made from blocks by machining. The molds are closed and filled with sufficient molten lead to fill the mold, leaving some excess to form a sprue, which is then removed by cutting or stamping. Grids can also be formed by mechanical working, either by cutting deep grooves into a sheet of steel, or by rolling up crimped strips and inserting them into holes in a cast plate, see Metal Forming TLP.
Lead Oxide
The lead can be oxidised by two processes: The Barton pot and the ball mill.
- Barton pot: A fine stream of molten lead is inserted into a heated vessel. Each droplet reacts with the air to form an oxide layer, giving 70 – 85% lead oxide.
- Ball milling: Pieces of lead are put into a rotary mechanical mill, forming fine lead flakes, which are then oxidised in air and removed. This also gives 75 – 80% lead oxide.
Red lead (Pb3O4) can also be added to the PbO formed by these methods, as it is more conductive. This is produced from PbO by roasting in a flow of air. This process would also increase the percentage of lead oxide in the material.
The oxide is mixed with water, sulphuric acid and a mixer, and then mixed to form a paste. It is then integrated with the grid by extrusion to form a plate. The paste is pressed by a machine into the interstices of the grid. They are partially dried, then stacked for curing. The curing process transforms the paste to a cohesive, porous solid. The most typical form of curing is “hydrosetting”: the grid is left at low temperature and humidity (25 – 40°C and 8 – 20% H2O) for between 24 and 72 hours.
Assembly
The simplest cell would consist of one cathode plate, one anode plate and a separator between them. In practice, most cells contain up to 30 plates with separators between. The separators are usually cellulose, PVC, rubber, microporous polyethylene or non-woven polypropylene. The plates are stacked and welded together. The tabs that are fixed to the plates are cast, then punched on between the layers and welded together. The plates are suspended inside the case, which is filled with electrolyte in order to activate it.


